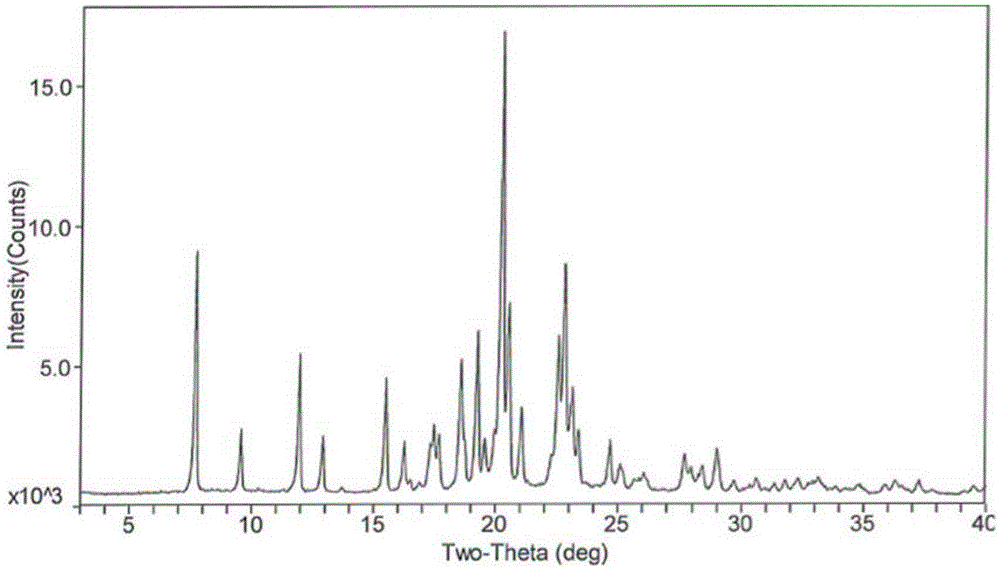
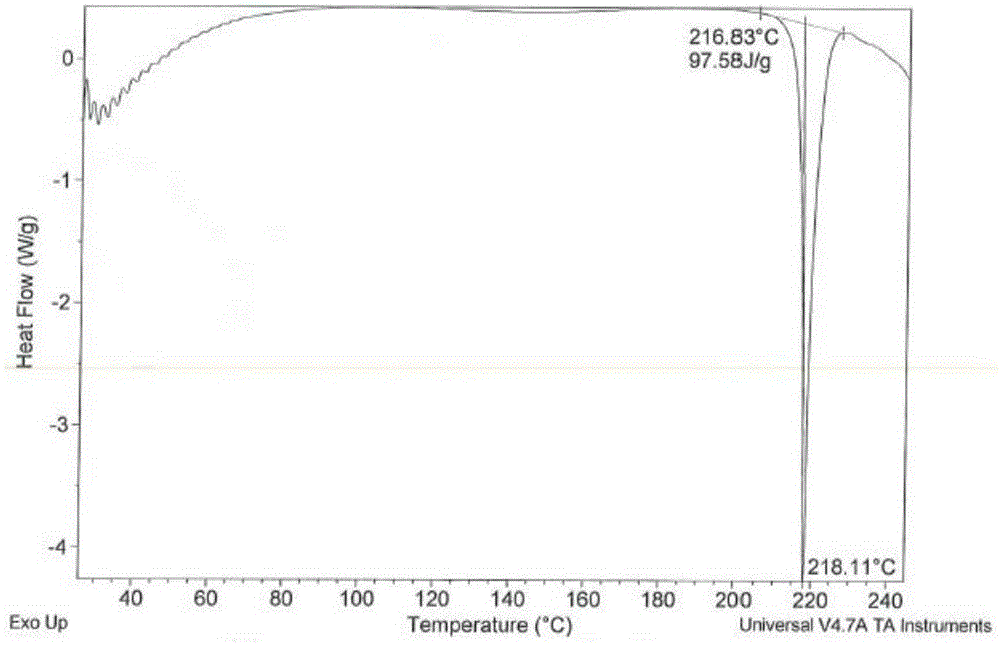
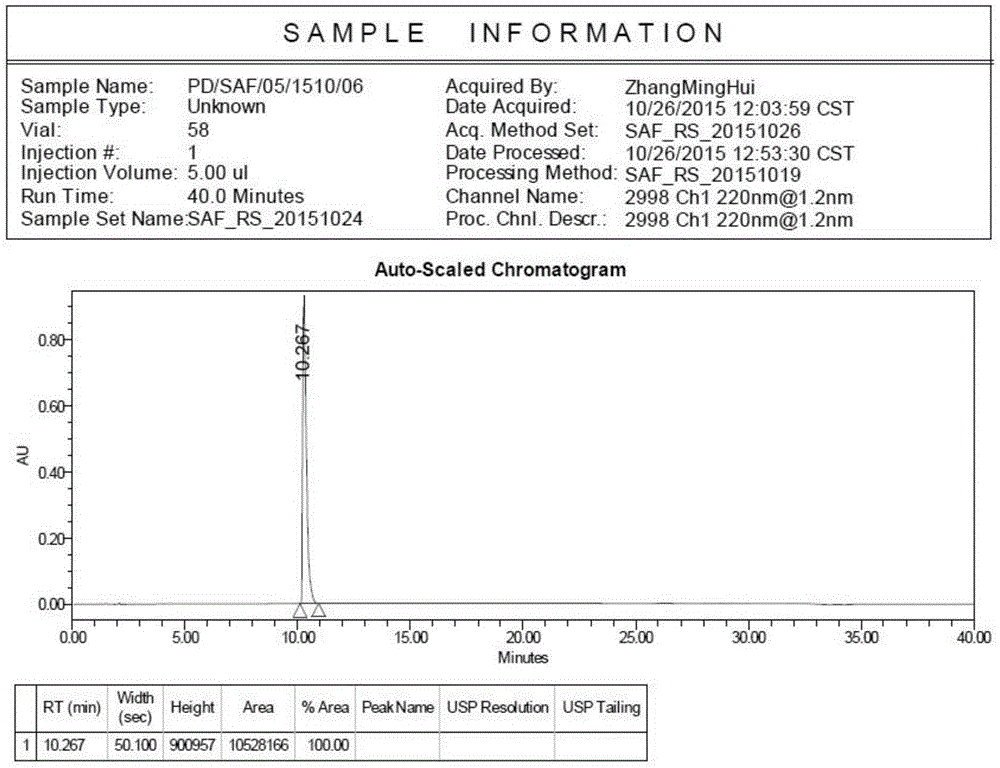
Preparation method of safinamide mesilate A1 crystal form
A technology of safinamide methanesulfonate and safinamide, which is applied in the preparation of sulfonate, carboxylic acid amide, preparation of organic compounds, etc., can solve the impurities of methanesulfonic acid safinamide A1 crystal form and optical difference. Problems such as high structure content and insufficient support
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation method of the safinamide methanesulfonate A1 crystal form of the present embodiment comprises the following steps:
[0027] (1) in N 2 Under protection, add 45mL of acetone to a 50mL three-neck flask, then cool down to 10°C, add 3.85g of methanesulfonic acid under stirring, transfer to a 100mL constant pressure dropping funnel for dropwise addition after stirring for 10min;
[0028] (2) in N 2 Under protection, add 11g of safinamide (BaseSAF-4) and 175mL of acetone to a 250mL four-neck flask at room temperature, then raise the temperature to 50°C under stirring, and stir for 30min until completely dissolved;
[0029] (3) Control the temperature at 50°C, add the solution of step (1) dropwise to the solution of step (2), and the dropwise addition time is about 1 to 2 hours. Crystallization at ℃ for 1h;
[0030] (4) After crystallization, filter, rinse the filter cake with 35 mL of acetone, and filter to dryness; take out the filter cake and dry it in va...
Embodiment 2
[0032] The preparation method of the safinamide methanesulfonate A1 crystal form of the present embodiment comprises the following steps:
[0033] (1) in N 2 Under protection, add 96mL of butanone into a 50mL three-necked flask, then cool down to 20°C, start adding 3.85g of methanesulfonic acid under stirring, transfer to a 100mL constant pressure dropping funnel for dropwise addition after stirring for 10min;
[0034] (2) in N 2 Under protection, add 11g of safinamide (BaseSAF-4) and 175mL of butanone to a 250mL four-neck flask at room temperature, then raise the temperature to 40°C under stirring, and stir for 30min until completely dissolved;
[0035] (3) Control the temperature at 40°C, add the solution of step (1) dropwise to the solution of step (2), and the dropwise addition time is about 1 to 2 hours. After the dropwise addition, keep stirring at the same temperature for 1.5 hours, and cool down to 20 Crystallization at ℃ for 1h;
[0036] (4) After crystallization, ...
Embodiment 3
[0038] The preparation method of the safinamide methanesulfonate A1 crystal form of the present embodiment comprises the following steps:
[0039] (1) in N 2 Under protection, add 45mL of methyl isobutyl ketone into a 50mL three-necked flask, then cool down to 15°C, add 4.12g of methanesulfonic acid under stirring, transfer to a 100mL constant pressure dropping funnel after stirring for 10min add;
[0040] (2) in N 2 Under protection, add 11g of safinamide (BaseSAF-4) and 330mL of methyl isobutyl ketone to a 250mL four-necked flask in sequence at room temperature, then raise the temperature to 45°C under stirring, and stir for 30min until completely dissolved;
[0041] (3) Control the temperature at 45°C, add the solution of step (1) dropwise to the solution of step (2), and the dropwise addition time is about 1 to 2 hours. After the dropwise addition, keep stirring at the same temperature for 1.5 hours, and cool down to 25 Crystallization at ℃ for 1h;
[0042] (4) After c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com