Visible light response with mesoporous structure cu 3 b 2 o 6 preparation method
A mesoporous structure, cu3b2o6 technology, applied in the field of Cu3B2O6 preparation, can solve the problem of low specific surface area of the product, and achieve the effects of simple and easy preparation method, reduced production cost and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Weigh 1.0g of copper acetate (Cu(CH 3 COO)2 ·H 2 (2, 5mmol) was dissolved in 10mL distilled water to prepare 0.5mol / L copper acetate solution; mmol) was dissolved in 10 mL of absolute ethanol to prepare an ethanol solution containing P123; the ethanol solution containing P123 was added dropwise to the copper acetate solution, and stirred for 0.5 h to obtain a mixed solution. Dissolve 2 g of borax (5 mmol) in 10 mL of deionized water, add the resulting borax aqueous solution to the aforementioned mixture, and stir magnetically for 1 hour to obtain a suspension, transfer the suspension to a reaction kettle, heat at 110°C for 12 hours, and obtain the product After centrifugation, washing with deionized water at 60°C (150mL×4) and absolute ethanol (50mL×3), the washed product was dried at 110°C for 12h, and then roasted at 550°C for 7h in an air atmosphere. (The heating rate is 5°C / min), and the dark green powder Cu 3 B 2 o 6 0.43g.
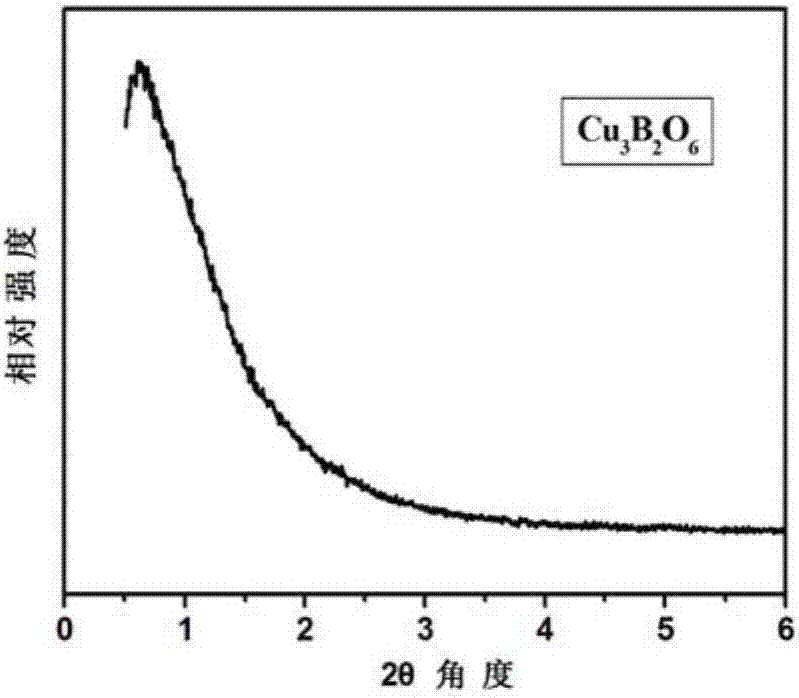
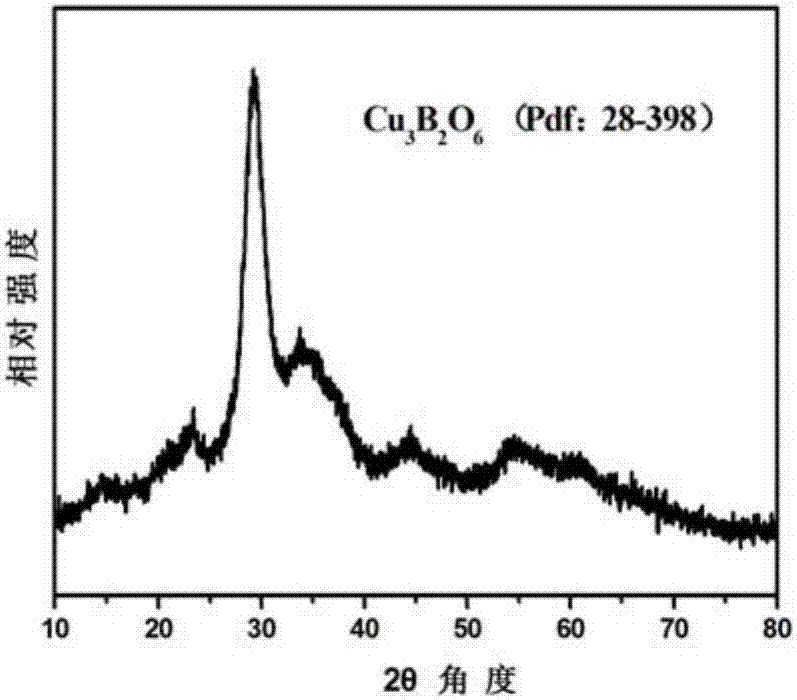
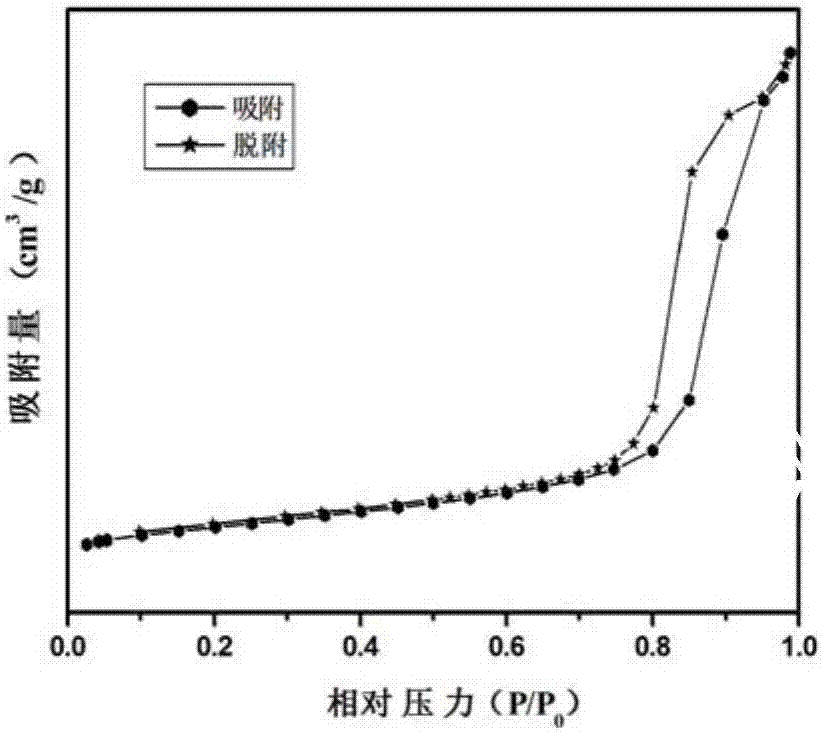
[0035] Tested by small angle XRD,...
Embodiment 2
[0037] Weigh 0.605g of copper nitrate (Cu(NO 3 ) 2 ·3H 2 (2.5mmol) was dissolved in 5mL distilled water to prepare 0.5mol / L copper nitrate solution; 0.25g polyethylene oxide-polypropylene oxide-polyethylene oxide triblock copolymer (F127, molecular weight 12600, 0.02 mmol) was dissolved in 5 mL of absolute ethanol to prepare an ethanol solution containing F127, which was added dropwise to the copper nitrate solution and stirred for 1 h to obtain a mixed solution. Dissolve 0.155g of boric acid (2.5mmol) in 5mL of deionized water, add the obtained aqueous solution of boric acid to the aforementioned mixture, stir magnetically for 1 hour to obtain a suspension, transfer the suspension to a reaction kettle, heat at 90°C for 72 hours, The obtained product was centrifuged, washed with deionized water at 6°C (150mL×4), and washed with absolute ethanol (50mL×4). After the washed product was dried at 90°C for 72h, the temperature was programmed to rise to 450°C in an air atmosphere. ...
Embodiment 3
[0040] Weigh 1.5g of copper acetate (Cu(CH 3 COO) 2 ·H 2 (2, 7.5mmol) was dissolved in 30mL distilled water to prepare 0.25mol / L copper acetate solution; mmol) was dissolved in 20 mL of absolute ethanol to prepare an ethanol solution containing P123, and the ethanol solution containing P123 was added dropwise to the copper acetate solution, and stirred for 3 h to obtain a mixed solution. Dissolve 1.726g of n-butyl borate (7.5mmol) in 10mL of absolute ethanol, add the obtained ethanol solution of n-butyl borate to the aforementioned mixture, stir magnetically for 1 hour to obtain a suspension, and transfer the suspension to the reaction kettle In 170°C water heating for 2 hours, the obtained product was centrifuged, washed with deionized water at 8°C (100mL×3), and washed with absolute ethanol (80mL×3). After drying the washed product at 120°C for 72h, it was Then, the temperature was programmed to rise to 650°C for 2 hours (the heating rate was 1°C / min), and the dark green ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com