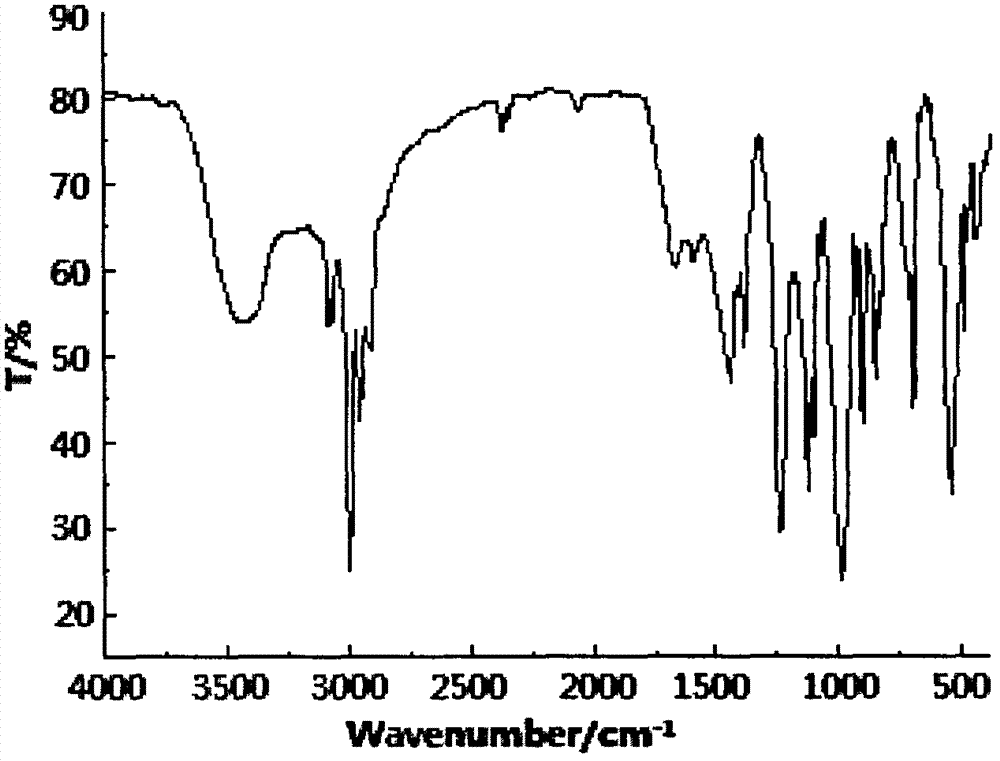
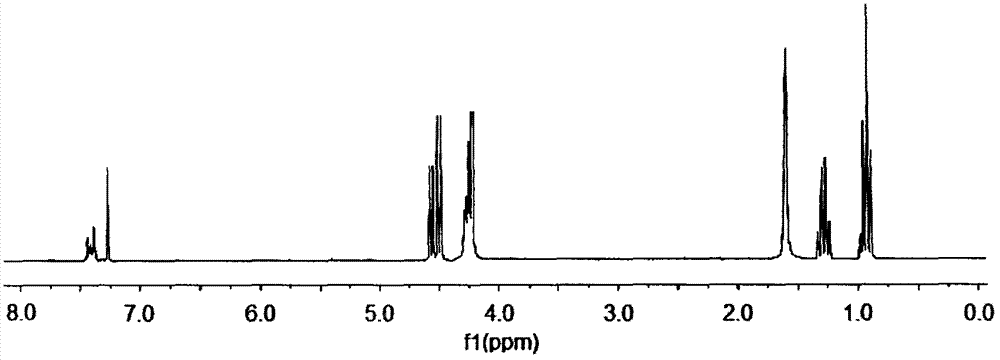
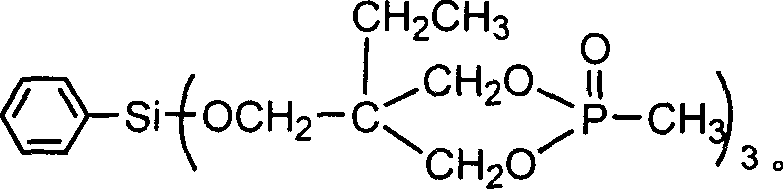
Preparation method of aryl silicon ring phosphine compound
A phosphine compound and compound technology, which is applied in the field of preparation of flame retardant phenyltrisilane compound, can solve the problems of poor electrical performance, high price, and restricted development, and achieve the promotion of flame retardant effect, small mechanical properties, and prevention of molten drops fall effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 In a 200ml four-necked flask equipped with a stirrer, a thermometer, a high-efficiency reflux condenser and a hydrogen chloride absorption device, replace the air in the bottle with nitrogen, and add 60ml of ethylene glycol diethyl ether and 34.92g (0.18mol) of 4- Hydroxymethyl-4-ethyl-cyclic methyl phosphonate, add 10.58g (0.05mol) phenyltrichlorosilane dropwise under stirring, control the dropping temperature not to exceed 60°C, and raise the temperature to 110°C after dropping, React for 19 hours, after the hydrogen chloride is released, change it to a vacuum distillation device, and remove ethylene glycol diethyl ether (recycled) by vacuum distillation, then add 55ml of distilled water, stir to disperse the solid in water, suction filter, and rinse with water until pH = 7, drying to obtain a white solid aryl silicon cyclic phosphine compound, the product yield is 90.5%.
Embodiment 2
[0024] Example 2 In a 200ml four-neck flask equipped with a stirrer, a thermometer, a high-efficiency reflux condenser and a hydrogen chloride absorption device, replace the air in the bottle with nitrogen, and add 70ml of xylene and 31.04g (0.16mol) of 4-hydroxymethyl -4-Ethyl-cyclic methyl phosphonate, add 10.58g (0.05mol) phenyltrichlorosilane dropwise under stirring, control the dropping temperature not to exceed 60°C, raise the temperature to 100°C after dropping, react for 21h, After the hydrogen chloride is discharged, change it to a vacuum distillation device, and remove xylene by vacuum distillation (recycling), then add 70ml of distilled water, stir to disperse the solid in water, filter with suction, wash with water until pH = 7, and dry , to obtain a white solid aryl silicon cyclic phosphine compound with a product yield of 84.2%.
Embodiment 3
[0025] Example 3 In a 200ml four-neck flask equipped with a stirrer, a thermometer, a high-efficiency reflux condenser and a hydrogen chloride absorption device, replace the air in the bottle with nitrogen, and add 50ml of diethylene glycol dimethyl ether and 32.98g (0.17mol) 4-Hydroxymethyl-4-ethyl-cyclic methylphosphonate, add 10.58g (0.05mol) phenyltrichlorosilane dropwise under stirring, control the dropping temperature not to exceed 60°C, and raise the temperature to 150°C after dropping ℃, reacted for 13 hours, after the hydrogen chloride was released, it was changed to a vacuum distillation device, diethylene glycol dimethyl ether (recycled) was removed by vacuum distillation, and then 60ml of distilled water was added, stirred to disperse the solid in water, suction filtered, Rinse with water to pH = 7, and dry to obtain a white solid arylsilyl phosphine compound with a product yield of 86.0%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| limiting oxygen index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com