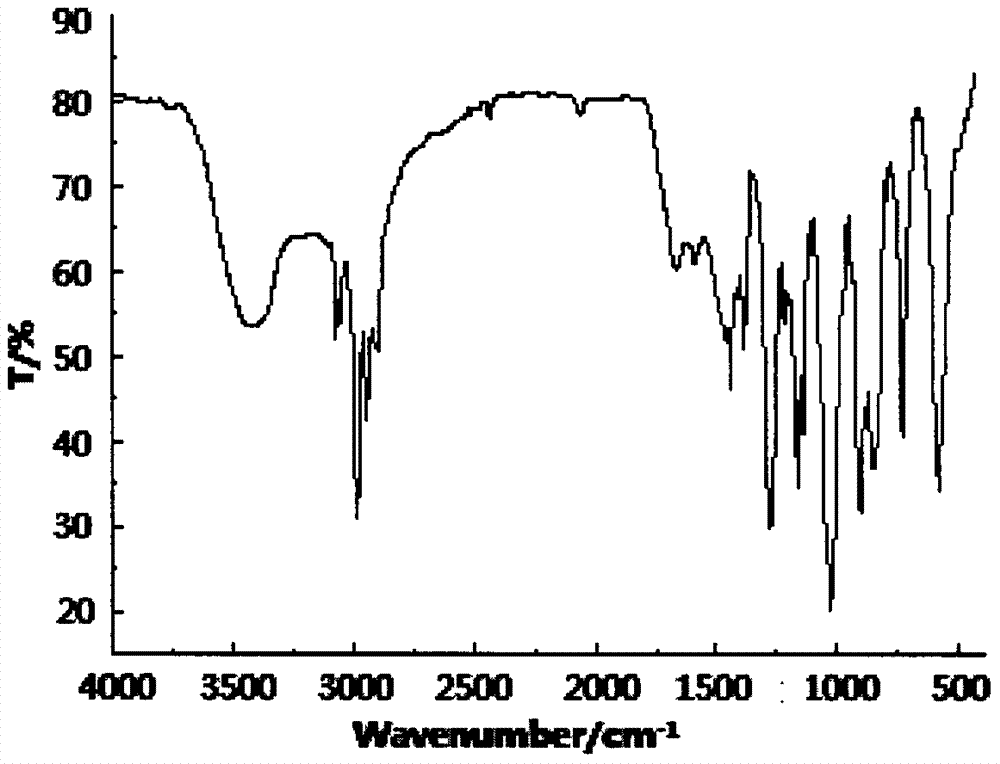
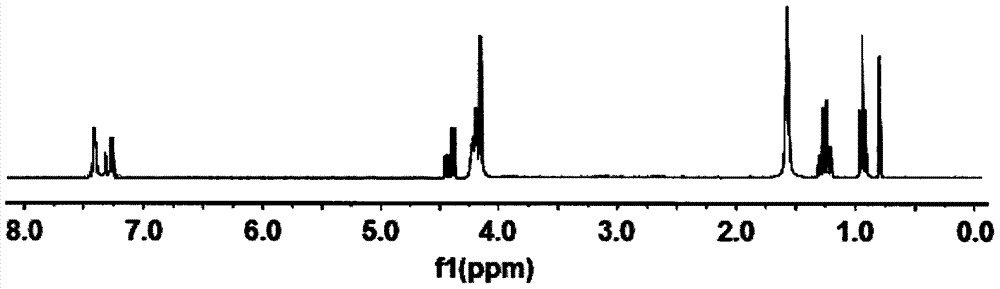
Methylphenyl methoxy phosphine heterocyclic methyl silicate compound and preparation method thereof
A technology of methyl phenyl methoxy phosphine silicate and heterocyclic methyl ester, which is applied in the field of methyl phenyl methoxy phosphine silicate heterocyclic methyl ester compound and its preparation, and can solve the problem of unsatisfactory material compatibility , application scope limitation, high volatility and other problems, to achieve the effect of promoting flame retardant effect, high atomic utilization rate, and no three waste emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 In a 200ml four-neck flask equipped with a stirrer, a thermometer, and a high-efficiency reflux condenser, replace the air in the bottle with nitrogen, and add 16.21g (0.10mol) of caged phosphate ester and 80ml of diethylene glycol dimethyl ether , 21.88g (0.12mol) methylphenyldimethoxysilane and 0.40g dimethyl sulfate, heat up to 160°C, keep the temperature for 13h, then cool to 50°C, transfer to a separatory funnel to stand for layering, Separate the lower layer of feed liquid, then add 35ml of toluene to wash, let stand to separate the lower layer of feed liquid, and then distill under reduced pressure to remove toluene and a small amount of low boiling point substances to obtain a light yellow liquid methyl phenyl methoxyphosphine heterocyclic methyl silicate , product yield 86.0%, its flash point (open cup): 207±5°C, decomposition temperature: 302±5°C, density (25°C): 1.734g / cm 3 , Refractive index: n D 25 = 1.4725.
Embodiment 2
[0029] Example 2 In a 100ml four-necked flask equipped with a stirrer, a thermometer, and a high-efficiency reflux condenser, the air in the bottle was replaced with nitrogen, and 16.21g (0.10mol) of caged phosphate, 20.05g (0.11mol) of methyl Phenyldimethoxysilane and 0.49g dimethyl sulfate, heat up to 170°C, keep warm for 11h, then cool to 50°C, add 35ml of toluene to wash, let stand to separate the lower layer of material liquid, and then distill under reduced pressure to remove toluene and A small amount of low-boiling matter, to obtain light yellow liquid methyl phenyl methoxyphosphonomethyl heterocyclic methyl silicate, the product yield is 84.6%, its flash point (open cup): 207±5°C, decomposition temperature: 302±5°C , Density (25°C): 1.734g / cm 3 , Refractive index: n D 25 = 1.4725.
Embodiment 3
[0030]Example 3 In a 200ml four-necked flask equipped with a stirrer, a thermometer, and a high-efficiency reflux condenser, replace the air in the bottle with nitrogen, and add 16.21g (0.10mol) of caged phosphate ester, 60mlDMF, 25.52g (0.14mol) Methylphenyldimethoxysilane and 0.35g methyl p-toluenesulfonate, heat up to 140°C, keep warm for 14h, then cool to 50°C, transfer to a separatory funnel to stand for stratification, and separate the lower layer of material liquid , then add 35ml of toluene to wash, let stand to separate the lower layer of feed liquid, then distill under reduced pressure to remove toluene and a small amount of low boiling point, to obtain light yellow liquid methyl phenyl methoxy phosphine heterocyclic methyl silicate, the product yield is 90.3 %, its flash point (open cup): 207±5°C, decomposition temperature: 302±5°C, density (25°C): 1.734g / cm 3 , Refractive index: n D 25 = 1.4725.
PUM
| Property | Measurement | Unit |
|---|---|---|
| flash point | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com