Synthetic method of dimethyl cysteamine hydrochloride
A technology of dimethylcysteine hydrochloride and a synthetic method, which is applied in the directions of thiol preparation, organic chemistry, etc., can solve the problems of low product yield, complicated operation, toxicity and the like, and achieves high product yield and high operation Simple, low-cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The method for synthesizing dimethylcysteine hydrochloride involved in the present invention is characterized in that the method uses isobutyraldehyde as a starting material and is prepared through condensation, reduction and ring-opening reactions. The method comprises the following process steps:
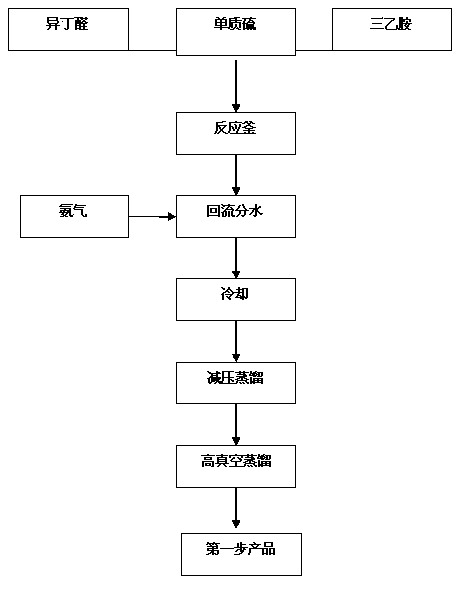
[0026] Step 1. Condensation
[0027] Add sulfur powder and triethylamine to isobutyraldehyde, raise the temperature to 60~65°C, feed ammonia gas, keep reflux and separate water, when the temperature of the reaction solution rises to 125~130°C, the reaction will generate 5,5-di Methyl-2-isopropylthiazoline, after the reaction, the system was distilled under reduced pressure to collect 5,5-dimethyl-2-isopropylthiazoline fractions. The molar ratio of isobutyraldehyde, elemental sulfur, triethylamine and ammonia is 2:1:0.1:1.5-2.5.
[0028] Its reaction formula is:
[0029]
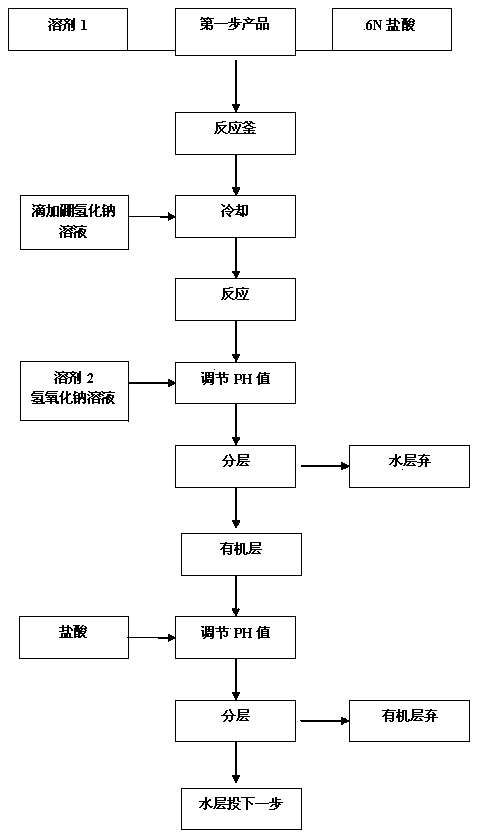
[0030] Step 2. Restoration
[0031] Add 5,5-dimethyl-2-isopropylthiazoline into organic solven...
Embodiment 1
[0043] Step 1. Condensation
[0044] Add 1.11kg of sulfur powder and 347g of triethylamine into 5kg of isobutyraldehyde, heat up to 60~65°C, feed ammonia gas, keep reflux and divide water, when the temperature of the reaction solution rises to 125~130°C, the reaction produces 5 , 5-dimethyl-2-isopropylthiazoline, after the reaction, the system was distilled under reduced pressure to collect 5,5-dimethyl-2-isopropylthiazoline fractions. 4.28 kg of 5,5-dimethyl-2-isopropylthiazoline was obtained with a gas phase purity of 96.7% and a yield of 78.5%.
[0045] Step 2. Restoration
[0046] Add 4kg of 5,5-dimethyl-2-isopropylthiazoline into 16kg of methanol, cool down to -5°C, add 24kg of 6N hydrochloric acid at -5-0°C, add sodium borohydride solution dropwise at -5-0°C After dropping, react at -5-0°C for 1h, reduce 5,5-dimethyl-2-isopropylthiazoline to generate 5,5-dimethyl-2-isopropylthiazolidine, add formaldehyde to the system after reaction base tert-butyl ether, sodium hydro...
Embodiment 2
[0051] Step 1. Condensation
[0052] Same as example 1.
[0053] Step 2. Restoration
[0054] Add 4kg of 5,5-dimethyl-2-isopropylthiazoline into 16kg of methanol, cool down to -5°C, add 24kg of 6N hydrochloric acid at -5-0°C, add sodium borohydride solution dropwise at -5-0°C , after dropping, react at -5-0°C for 1h, reduce 5,5-dimethyl-2-isopropylthiazoline to generate 5,5-dimethyl-2-isopropylthiazolidine, add acetic acid to the system after reaction Ethyl ester and sodium hydroxide to adjust the pH value to 7~8, separate the liquid, discard the aqueous phase, add water and hydrochloric acid to the organic phase to adjust the pH value to 2~3, separate the liquid, discard the organic phase, and the aqueous layer contains 5,5-dimethyl -2-Isopropylthiazolidine cast directly to the next step.
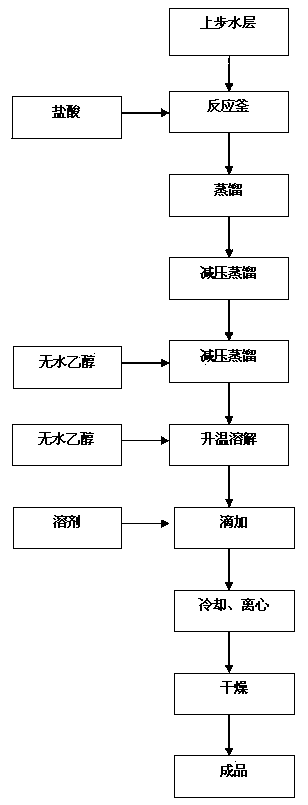
[0055] Step 3, open loop
[0056] The water layer containing 5,5-dimethyl-2-isopropylthiazolidine is heated to reflux, and the water and the isobutyraldehyde formed by the reaction are d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com