Fluorosilicone functional macromonomer with alkene double bond on single end and preparation method thereof
A technology of macromonomer and olefinic double bond, which is applied in the field of fluorine-silicon functional macromonomer containing olefinic double bond at one end and its preparation field, which can solve the problem of less application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) After evacuating the polymerization bottle to high vacuum for 1 hour, add F 3 Monomer (34.3ml, 0.091mol), 40% (Vol) THF (290ml) and n-butyllithium 37.5ml (2.4M n-hexane solution), reacted for 2h to prepare oxyanion initiator lithium silanolate, and then added the remaining F 3 Monomer (343.2ml, 0.909mol) was subjected to ring-opening polymerization reaction for 3h, and then 19.3ml of end-capping agent was added to end-cap for 12h.
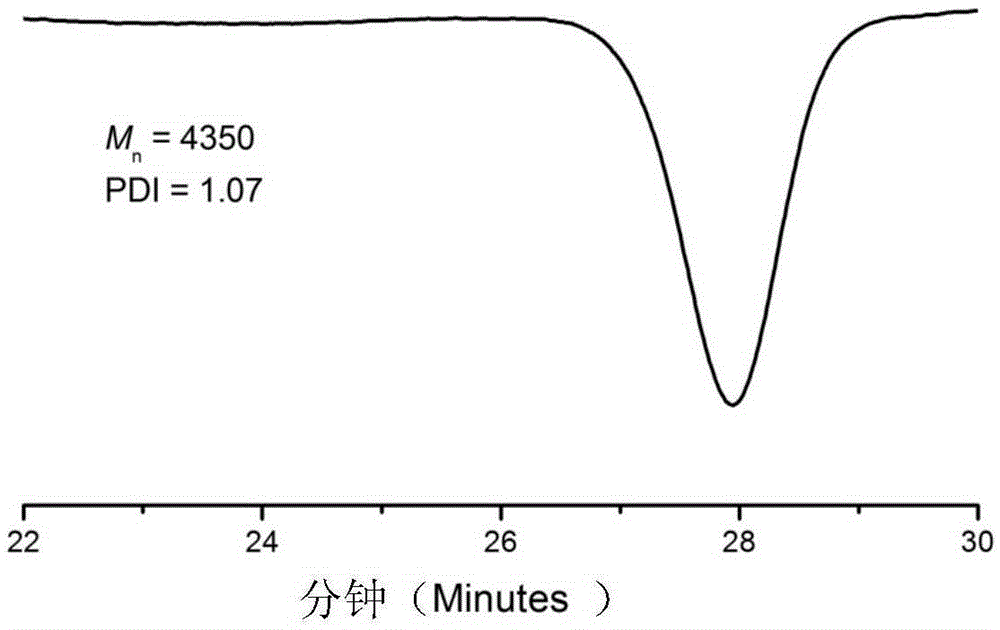
[0037] (2) After the product was centrifuged, it was distilled under reduced pressure to remove impurities such as solvents and unreacted monomers, and the product was washed with methanol and dried in a vacuum oven for 24 hours. For products 1 HNMR and gel permeation chromatography (GPC) characterize the structure, molecular weight and distribution of functional macromolecules, in which the gel permeation chromatogram is as follows figure 1 As shown, through the above characterization, it is shown that the product is a structure of fo...
Embodiment 2
[0040] (1) After evacuating the polymerization bottle to high vacuum for 1 hour, add F 3 Monomer (34.3ml, 0.091mol)), 40% (Vol) (290ml) THF and n-butyllithium 37.5ml (2.4M n-hexane solution), reacted for 2h to prepare oxyanion initiator lithium silanolate, and then added the remaining f 3 Monomer (343.2ml, 0.909mol) was subjected to ring-opening polymerization reaction for 3h, and then 21.4ml of end-capping agent was added to end-cap for 12h.
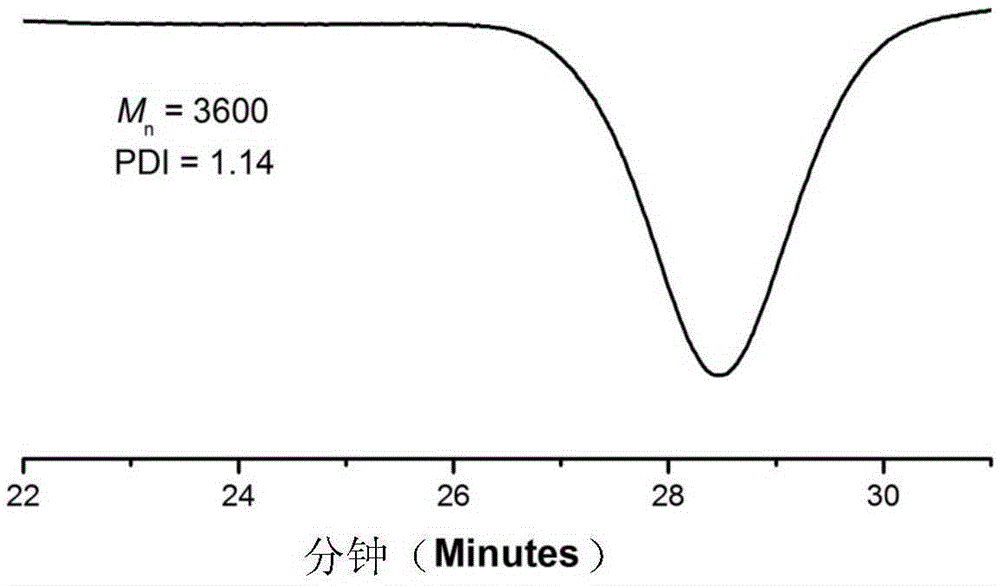
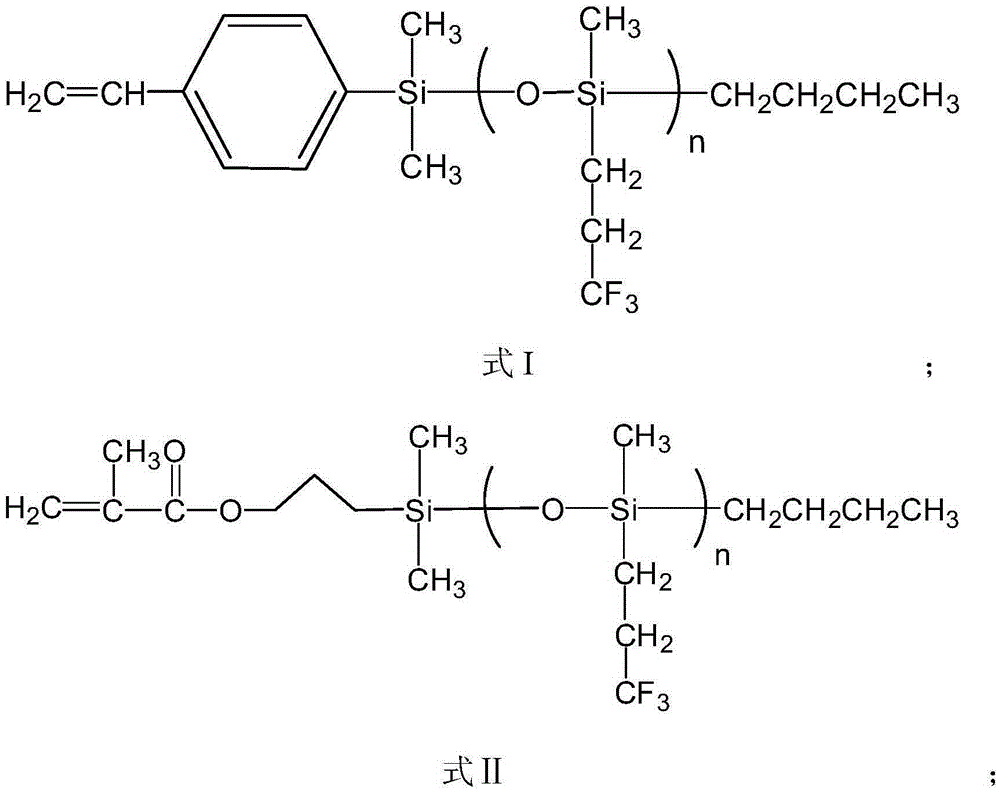
[0041] (2) After the product was centrifuged, it was distilled under reduced pressure to remove impurities such as solvents and unreacted monomers, and the product was washed with methanol and dried in a vacuum oven for 24 hours. For products 1 HNMR and GPC characterize the structure, molecular weight and distribution of functional macromolecules, among which the gel permeation chromatogram is as follows figure 2 As shown, through the above characterization, it is shown that the product has the structure of formula II, n=21.5.
[0...
Embodiment 3
[0044] (1) After evacuating the polymerization bottle to high vacuum for 2 hours, add F 3 Monomer (17.4ml, 0.046mol), 50% (Vol) (405ml) THF and n-butyllithium 18.8ml (2.4M n-hexane solution), reacted for 1h to prepare oxyanion initiator lithium silanolate, and then added the remaining F 3 Monomer (360.2ml, 0.954mol) was subjected to ring-opening polymerization reaction for 3h, and then 9.7ml of end-capping agent was added to end-cap for 12h.
[0045] (2) After the product was centrifuged, it was distilled under reduced pressure to remove impurities such as solvents and unreacted monomers, and the product was washed with methanol and dried in a vacuum oven for 24 hours. For products 1 The structure, molecular weight and distribution of the functional macromolecules were characterized by HNMR and GPC. Through the above characterizations, it was shown that the product had a structure of formula I, n=56.7.
[0046]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com