A tumor precision T cell containing an efficient killing mechanism and its use
A cell and tumor technology that can be used in the fields of immunology and cell biology to solve problems such as decreased therapeutic efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Example 1: Synthesis of CAR expression cassette and construction of expression vector
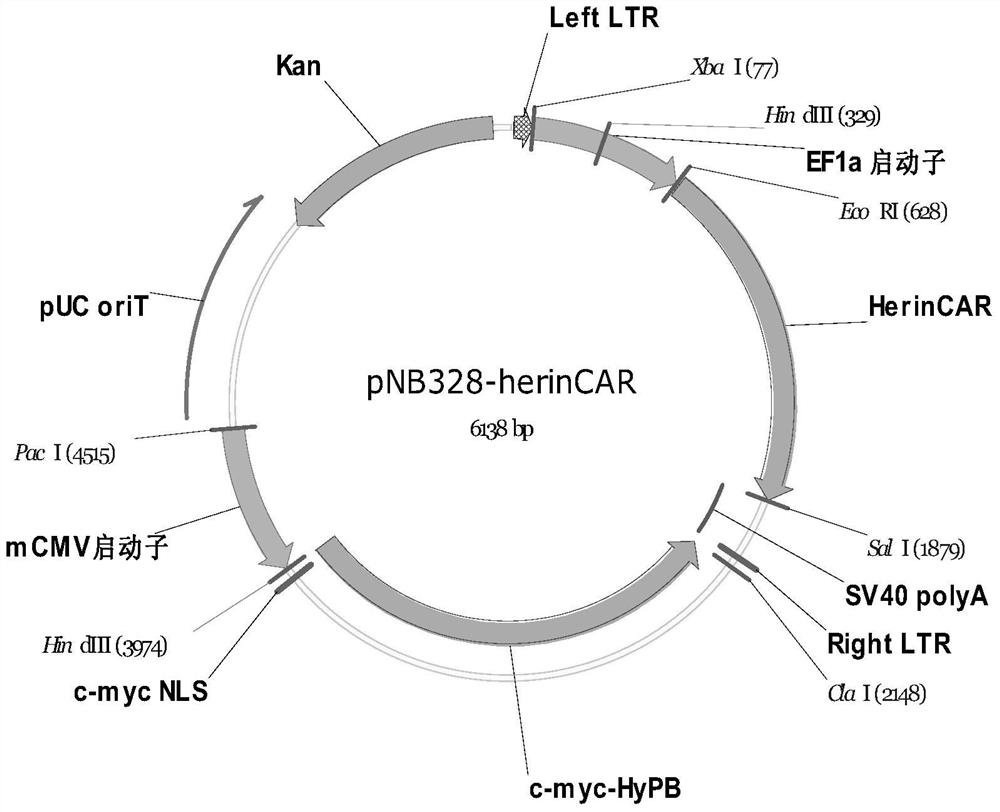
[0087] According to the composition structure of herinCAR (for the model diagram, see figure 1 ), spliced into the whole fusion amino acid sequence and coding DNA expression frame:
[0088] The amino acid residue sequence of herinCAR is:
[0089] GGGGGGGGG GGGGGGGGG FVPVFLPAKPTTTPAPRPPTPAPTIASQPLSLRPEACRPAAGGAVHTRGLDFACDIYIWAPLAGTCGVLLLSLVITLYCNHRNRFSVVKRGRKKLLYIFKQPFMRPVQTTQEEDGCSCRFPEEEEGGCELRVKFSRSADAPAYQQGQNQLYNELNLGRREEYDVLDKRRGRDPEMGGKPRRKNPQEGLYNELQKDKMAEAYSEIGMKGERRRGKGHDGLYQGLSTATKDTYDALHMQALPPR(SEQ ID NO:1)。
[0090] Among them, the signal peptide (MALPVTALLLPLALLLHAARPS, SEQ ID NO:3) is underlined with a dotted line, and the CD20 epitope (NIYNCEPANPSEKNSPSTQYCYSI, SEQ ID NO:4) recognized by the CD20 commercial antibody MatThera is underlined with a wavy line, and the linker sequence with a single underline. Bold is HERIN (GTHSLPPRAAVVPLRMQPGPAHPVLSFLRPS...
Embodiment 2
[0101] Example 2: Isolation and culture of cholangiocarcinoma tissue-derived TIL
[0102] Collect freshly resected cholangiocarcinoma specimens and process them immediately under sterile conditions. The specific method is as follows: remove the normal tissue and necrotic area around the cholangiocarcinoma specimen, and remove the 1-2mm in size from different areas of the specimen 3 Place a small tissue block in each well of a 24-well plate. Add 2 mL of complete medium (GT-T551 medium containing 10% FBS) and 3000 IU / mL IL-2 to each well. Place the 24-well plate at 37 °C, 5% CO 2 cultured in an incubator. On the 5th to 6th day after the initiation of culture, a half-volume medium change was performed for all wells. Afterwards, according to the growth of tumor infiltrating lymphocytes (tumor infiltrating lymphocytes, TIL), a half-volume liquid change was performed every 1-2 days. Once the wells were overgrown with TILs and all adherent cells had been removed, the TILs in e...
Embodiment 3
[0104] Example 3: Genetic Modification of TIL Cells
[0105] Collect 1×10 7 For TIL cells (prepared in Example 2), transfect 6 μg of the pNB328-herinCAR plasmid (prepared in Example 1) into the nucleus through a Lonza 2b-Nucleofector instrument, place at 37°C, 5% CO 2 Culture in an incubator; transfer to a 6-well plate containing 30ng / mL anti-CD3 antibody and 3000IU / mL IL-2 (purchased from Novoprotein Company) after 6 hours, and place at 37°C, 5% CO 2 Incubator culture. After the cells were confluent, they were subcultured at a ratio of 1:5. The TIL cells containing herinCAR, referred to as Bz-T cells, were obtained and used in the following Examples 4-7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com