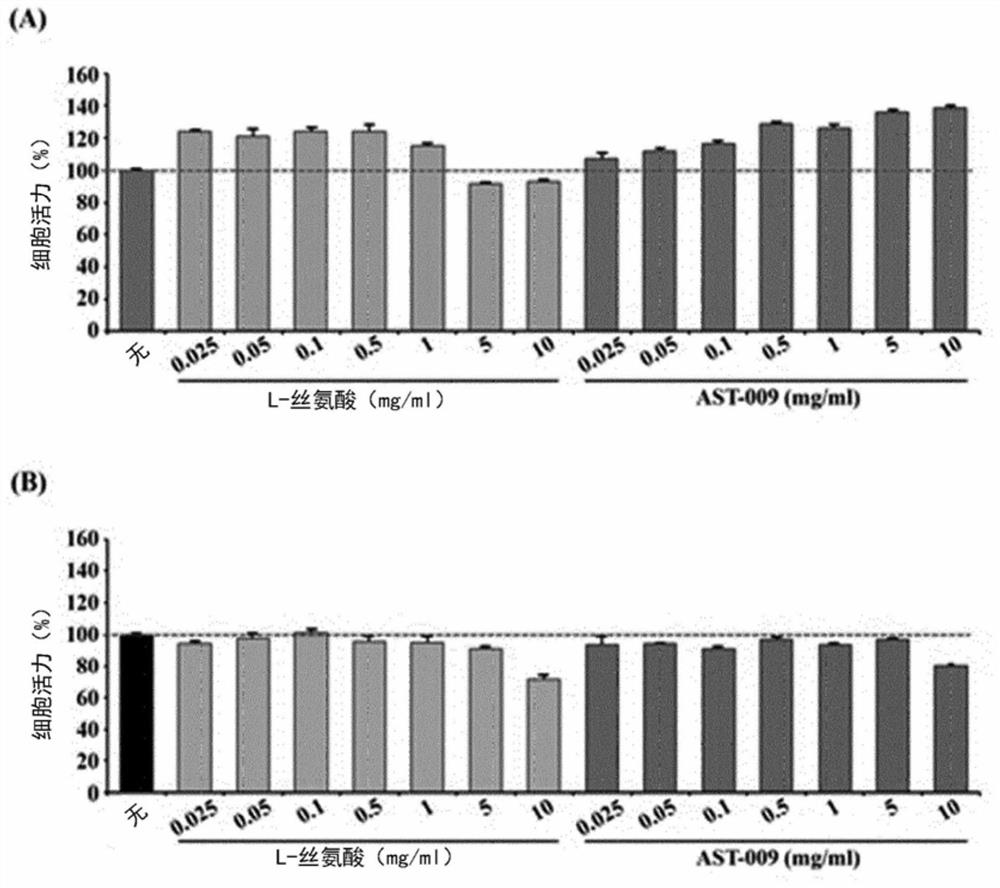
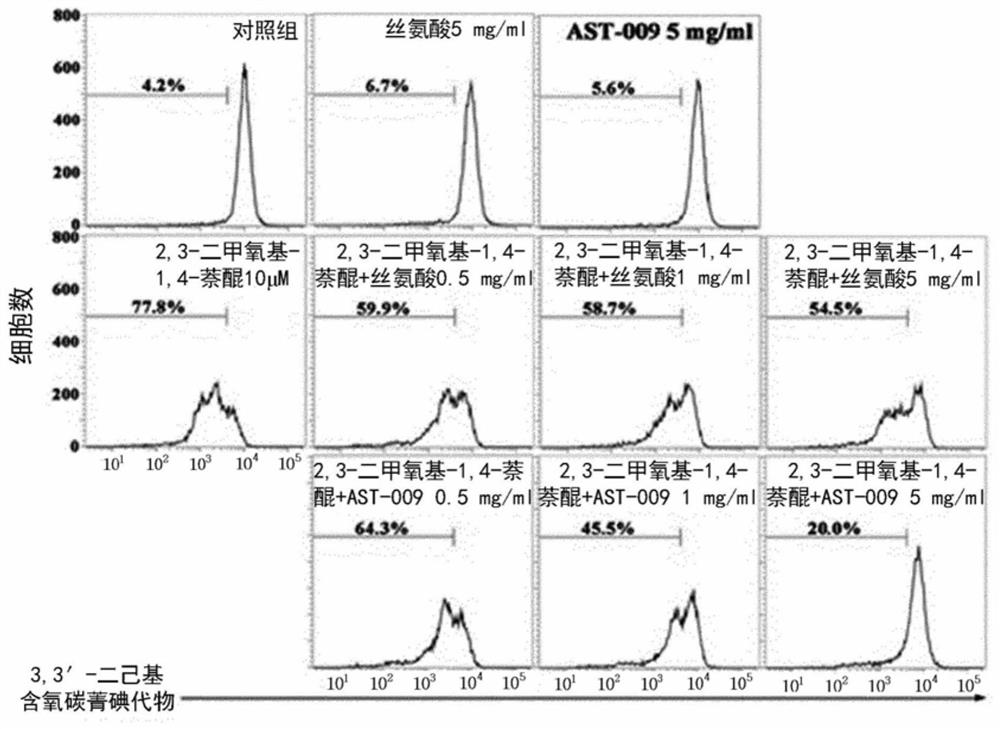
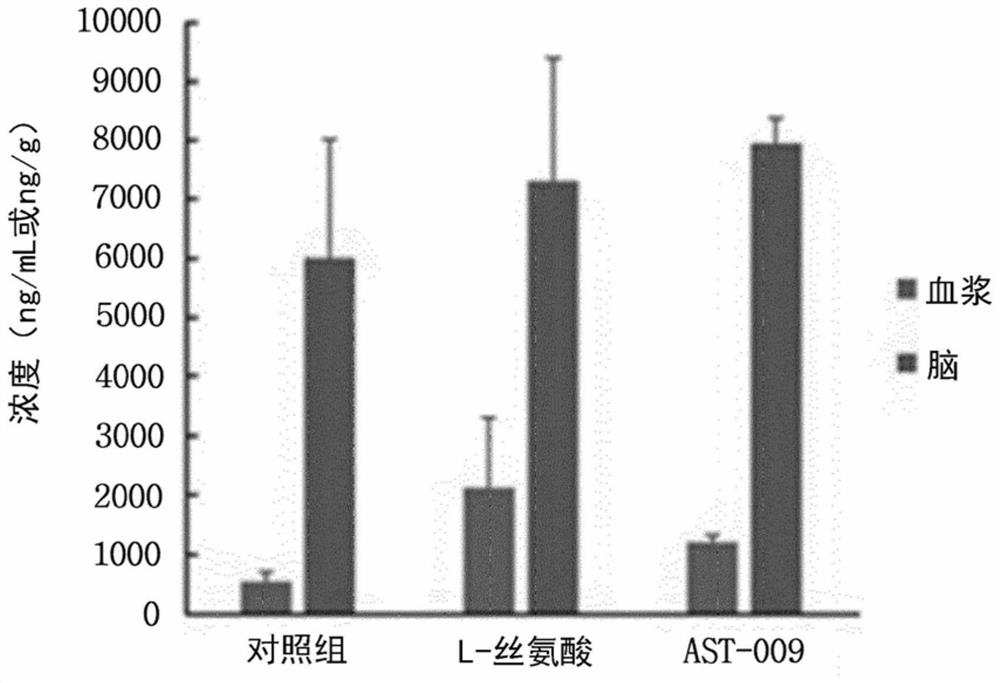
Serine derivative compound for preventing or treating central nervous system diseases
A technology of central nervous system and compound, applied in nervous system diseases, neuromuscular system diseases, muscular system diseases, etc., can solve problems such as low permeability, and achieve the effect of improving blood-brain barrier permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] Therefore, the present invention provides the preparation method of the compound of above-mentioned chemical formula (I), comprises introducing H-Ser(Trt)-OH to 2-chlorotrityl chloride resin (2-Chlorotrityl chloride resin) continuously 3 times, and introducing H - A step to cleave the resin after Lys(Boc)-OH.
[0060] Referring to the synthesis scheme (scheme), the specific preparation method of the serine derivative compound of chemical formula (I) is described as follows:
[0061] [Scheme]
[0062]
[0063] (1) CLTR Swelling and H-Ser(Trt)-OH introduction
[0064] 1) Add 2-chlorotrityl chloride resin (2-Chlorotrityl chloride resin) (0.1 mmol) and MC (0.4 L) to a 4 L reactor, and expand (swelling) for 2 hours.
[0065] 2) Add dimethylformamide (DMF) containing Fmoc-Ser(trt)-OH (1.5eq) and diisopropylethylamine (DIPEA) (3eq) to the resin (resin) of Swelling ( 0.2L), and reacted at room temperature for 6 hours.
[0066] 3) After the reaction, wash with DMF (0.2 L)...
Embodiment
[0135] 1. Materials and methods
[0136] 1.1. Medium and reagents
[0137] Dulbecco's Modified Eagle Medium (DMEM), fetal bovine serum (FBS), 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES, 4 Reagents for cell culture such as -(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid) and streptomycin-penicillin were purchased from Gibco BRL (Grand Island, USA).
[0138] 1.2. Preparation of a novel serine derivative compound (AST-009)
[0139]2-Chlorotrityl chloride resin (2-Chlorotrityl chloride resin) (0.1 mmol) and MC (0.4 L) were added to a 4 L reactor, and swelling (swelling) was carried out for 2 hours. Add Fmoc-Ser(trt)-OH (1.5eq) and DIPEA (3eq) in DMF (0.2L) to the resin (resin) of swelling (resin), and react at room temperature (RT) for 6 hours, and the reaction ends Thereafter, washing was performed with DMF (0.2 L). A mixed solution of MC / MeOH / DIPEA (255:30:15) was added, followed by capping shaking reaction at room temperature for 2 hours, followed by washin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com