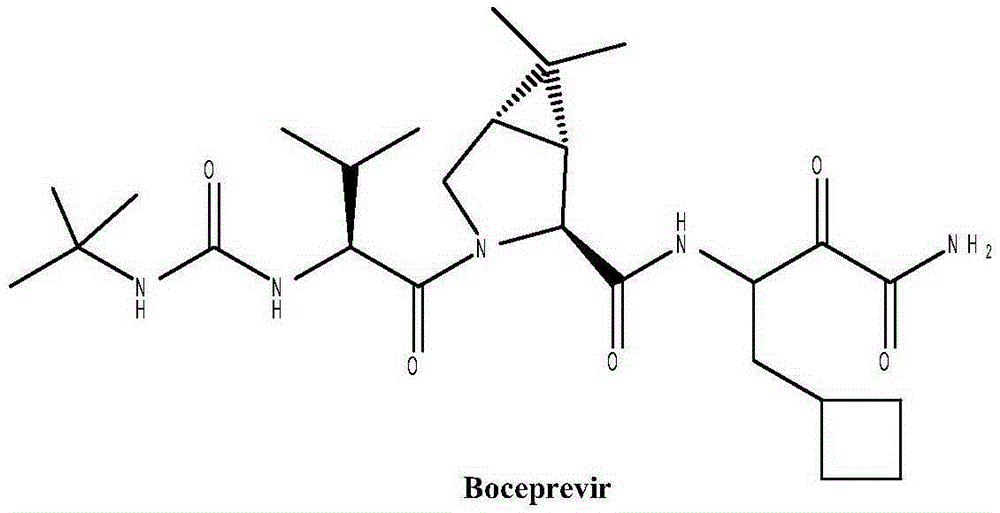
Preparation method of boceprevir intermediate
A boceprevir and intermediate technology, which is applied in the field of chemical drug intermediate preparation, can solve the problems of low yield, shortened synthetic route of target compound, and high cost of intermediates, and achieves easy availability of raw materials, low cost of raw materials, and synthetic route. short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
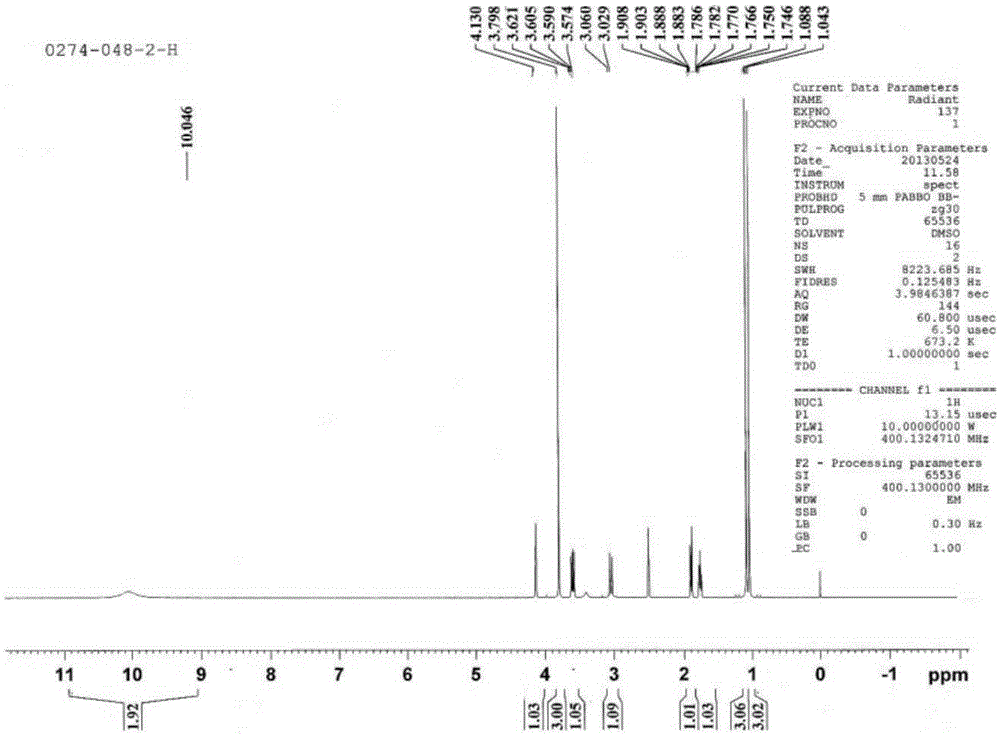
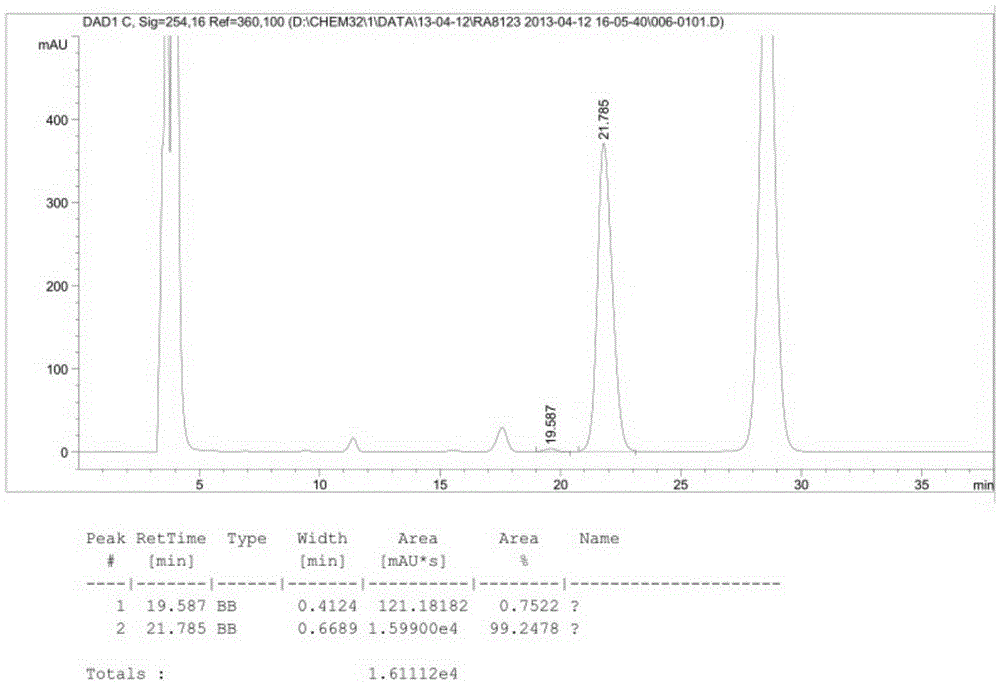
Image
Examples
Embodiment 1
[0034] Add 147.7g of 6,6-dimethyl-3-azabicyclo[3.1.0]hexane hydrochloride (1.0mol, 1.0eq) and 138g of potassium carbonate (1.0mol) into 1.5L of water, stir well, and then Slowly add 218.3 g of di-tert-butyl dicarbonate (1.0 mol) dropwise under an ice bath. After completion, warm up to room temperature and stir for 3 hours, stop stirring, let stand for liquid separation, and separate the upper organic layer, and the water layer with methyl tert-butyl Butyl ether (MTBE) was extracted once, the organic layers were combined, dried over anhydrous sodium sulfate, and concentrated to remove the solvent to obtain 205 g of intermediate A with a yield of 97.0%.
[0035] Add 200g of intermediate A (0.95mol, 1.0eq) to the three-necked flask successively, and add 108.5g of (1S,2S)-(+)-1,2-cyclohexanediamine (0.95mol, 1.0eq) as the chiral ligand body, add 2.0L methyl tert-butyl ether as anhydrous organic solvent, replace the air three times, N 2 For protection, cool the flask to -70°C with...
Embodiment 2
[0038] Add 147.7g of 6,6-dimethyl-3-azabicyclo[3.1.0]hexane hydrochloride (1.0mol) and 200.2g of potassium bicarbonate (2.0mol) into 1.5L of water, stir well, then ice Slowly add 256.0g of benzyl chloroformate (1.5mol) dropwise in the bath, after completion, rise to room temperature and stir for 3 hours, stop stirring, let stand for liquid separation, separate the upper organic layer, and extract the aqueous layer with MTBE once to obtain the organic phase , the organic layers were combined, dried using anhydrous sodium sulfate, and concentrated to remove the solvent to obtain 239.2 g of intermediate A with a yield of 97.5%.
[0039] Add 239g intermediate A (0.97mol, 1.0eq), 171.3g (1S,2S)-(+)-1,2-cyclohexanediamine (1.5mol) as chiral ligand and 2.0L Methyl tert-butyl ether, three air replacements, N 2 For protection, cool the three-necked flask to -70°C with liquid nitrogen, slowly add 1.07L of sec-butyllithium cyclohexane solution with a concentration of 1.4mol / L dropwise, ...
Embodiment 3
[0042] Add 147.7g of 6,6-dimethyl-3-azabicyclo[3.1.0]hexane hydrochloride (1.0mol) and 112.2g of potassium hydroxide (2.0mol) into 1.5L of water, stir well, then ice Slowly add 327.5 g of di-tert-butyl dicarbonate (1.5 mol) dropwise in the bath. After completion, rise to room temperature and stir for 3 hours, stop stirring, let stand for liquid separation, separate the upper organic layer, and extract the aqueous layer with MTBE once to obtain The organic phase was combined, dried using anhydrous sodium sulfate, and concentrated to remove the solvent to obtain 200 g of intermediate A with a yield of 94.7%.
[0043] Add 200g of intermediate A (0.95mol, 1.0eq) and 135.1g of chiral ligand (1S,2S)-(+)-N,N'-dimethyl-1,2-cyclohexanedi Amine (0.95mol, 1.0eq) and 2.0L tetrahydrofuran, three air replacements, N 2 For protection, cool the flask to -70°C with liquid nitrogen, and add dropwise 0.68L of 1.4mol / L sec-butyllithium cyclohexane solution, wherein the amount of sec-butyllithium...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com