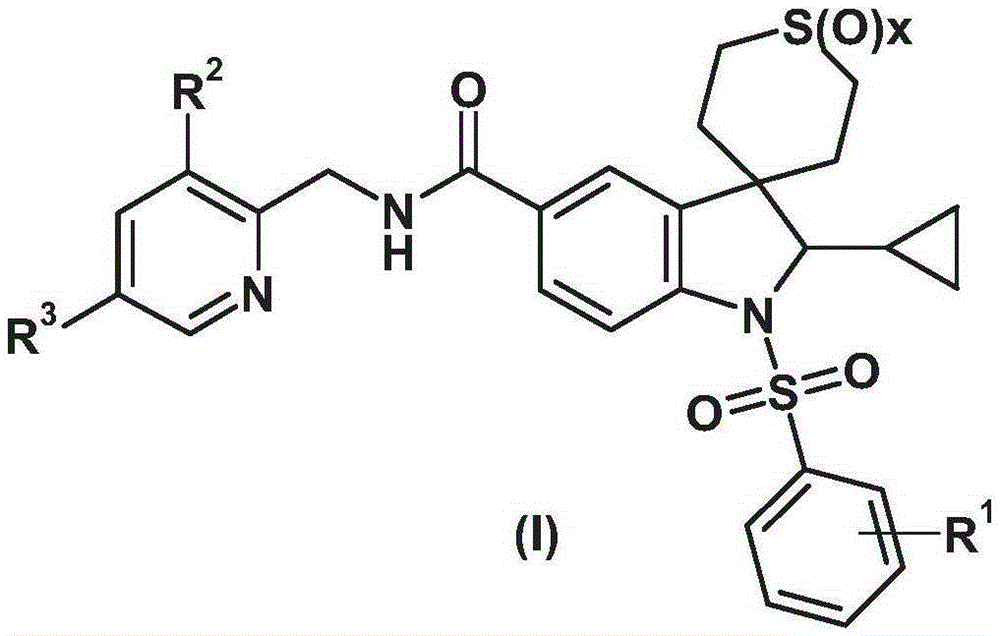
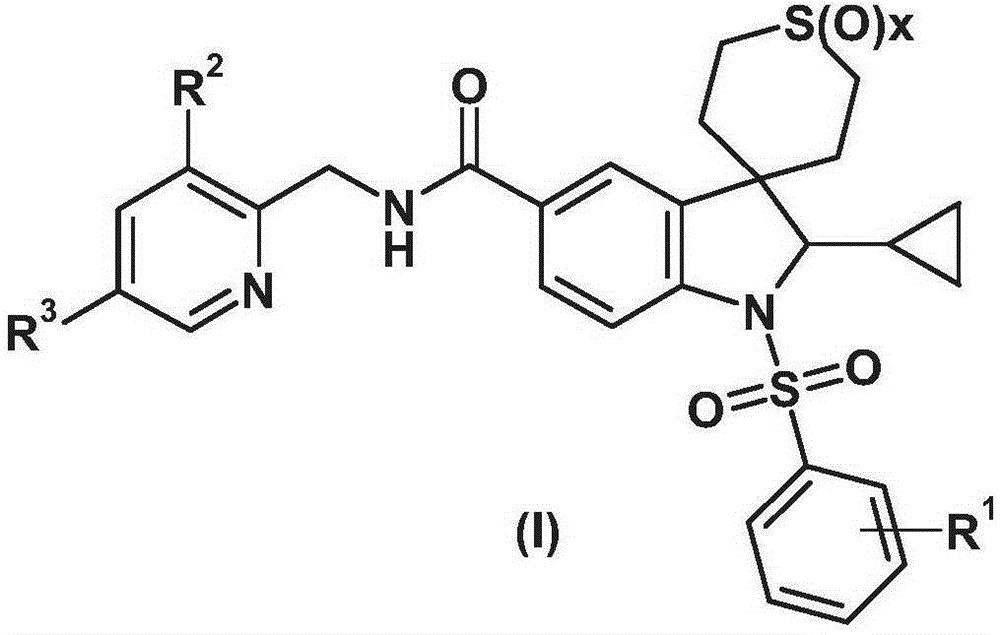
Spiroindoline derivatives for use as gonadotropin-releasing hormone receptor antagonists
A technology of indole and dioxide, applied in diseases, antineoplastic drugs, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0244] Preparation via Carbonyl Compounds: Step 1a Swern Oxidation
[0245] Preparation of 3,4,5,6-tetrahydro-2H-thiopyran-4-carbaldehyde
[0246]
[0247] 1.4 equivalents of oxalyl chloride (6.72 g, 52.9 mmol) were dissolved in 200 mL of dichloromethane, and the solution was cooled to -65°C. 2 equivalents of dimethylsulfoxide (5.91 g, 75.6 mmol) dissolved in 30 mL of dichloromethane were added dropwise over 10 minutes so that the temperature did not exceed -50°C. After 15 minutes, 1 equivalent of tetrahydrothiopyran-4-methanol (5.00 g, 37.8 mmol) dissolved in 30 mL of dichloromethane was added dropwise over 5 minutes at a temperature up to -45°C. The mixture was stirred for 1 h and warmed to -30 °C. 3 equivalents of triethylamine (11.5 g, 113 mmol) were added dropwise, then the mixture was allowed to warm to room temperature. After stirring for 1 h, the mixture was poured into water and extracted with dichloromethane. The combined organic phases were washed with water,...
Embodiment 1
[0424] 2-cyclopropyl-1-[(4-fluorophenyl)sulfonyl]-N-{[3-fluoro-5-(trifluoromethyl)pyridin-2-yl]methyl}-1,2, 2',3',5',6'-hexahydrospiro[indole-3,4'-thiopyran]-5-carboxamide 1',1'-dioxide
[0425]
[0426] With a slightly modified method to GP9.1: 100 mg (0.208 mmol) of intermediate F.1 and 83.5 mg (0.313 mmol, 1.5 equiv) of 1-[ 3-Fluoro-5-(trifluoromethyl)pyridin-2-yl]methylamine dihydrochloride was reacted with 119 mg (0.313 mmol, 1.5 equiv) of HATU in 3.5 mL of DMF at room temperature overnight to yield 122 mg (89%) desired amide. The crude product was not subjected to further purification. 1 H-NMR (300MHz, DMSO-d6): chemical shift [ppm] = 0.21-0.33 (m, 1H), 0.34-0.66 (m, 3H), 0.78-0.90 (m, 1H), 0.94-1.08 (m, 1H), 1.46(dt, 1H), 3.63(dt, 1H), 4.36(d, 1H), 4.69(d, 2H), 7.40(m, 2H), 7.58(m, 1H), 7.80-7.96(m, 4H), 8.28 (m, 1H), 8.79 (s, 1H), 9.14 (t, 1H). UPLC-MS(ESI+): [M+H] + =656.
[0427] The enantiomer of the racemic substance of Example 1 was passed through chiral p...
Embodiment 11
[0428] Example 1.1, (2S)-2-cyclopropyl-1-[(4-fluorophenyl)sulfonyl]-N-{[3-fluoro-5-(trifluoromethyl)pyridin-2-yl] Methyl}-1,2,2',3',5',6'-hexahydrospiro[indole-3,4'-thiopyran]-5-carboxamide 1',1'-dioxide: Rt=3.06min (enantiomer 1)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com