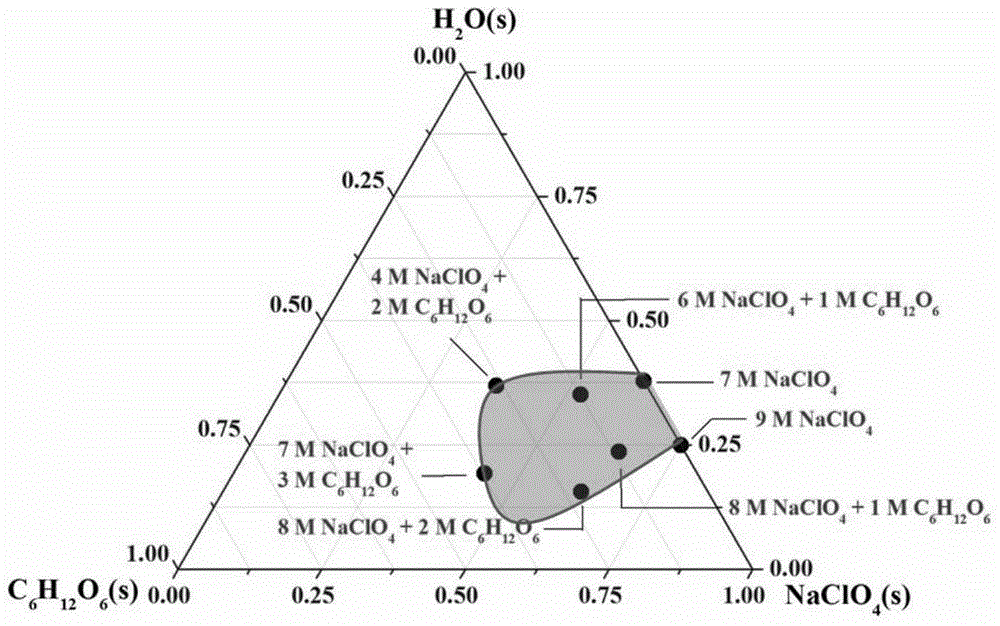
Aqueous electrolyte and super-capacitor
A water-based electrolyte and supercapacitor technology, which is applied in the direction of hybrid capacitor electrolyte, etc., can solve the problems of small operating temperature range of supercapacitor, the inability of the electrolyte to maintain the electrochemical properties, and the decrease of the conductivity of the electrolyte, and achieve good electrochemical performance, Not easy to freeze, the effect of large operating temperature range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The present invention has no special limitation on the preparation method of the supercapacitor, and preferably includes the following steps: uniformly mixing the electrode material and the conductive adhesive to obtain a mixture, and the conductive adhesive is preferably acetylene black and polytetrafluoroethylene;
[0039] coating the mixture on a metal plate and pressing it into a film to obtain a pole piece;
[0040] The two pole pieces are wound together with the diaphragm to form a core package, and placed in the electrolyte to obtain a supercapacitor, and the diaphragm is located between the two pole pieces.
Embodiment 1
[0044] 0.4mol sodium perchlorate (NaClO 4 ) and 0.05mol glucose (C 6 h 12 o 6 ) was dissolved in deionized water, then the solution was diluted to 100ml, and prepared to contain 4mol / LNaClO 4 , and at the same time contain 0.5mol / LC 6 h 12 o 6 aqueous electrolyte.
Embodiment 2
[0046] 0.5mol sodium perchlorate (NaClO 4 ) and 0.1mol glucose (C 6 h 12 o 6 ) was dissolved in deionized water, then the solution was diluted to 100ml, and prepared to contain 5mol / LNaClO 4 , and also contains 1mol / LC 6 h 12 o 6 aqueous electrolyte.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com