Preparation and applications of anti-Och1 monoclonal antibody
A monoclonal antibody, Candida albicans technology, applied in the field of medical bioengineering, can solve the problem of no anti-Och1 monoclonal antibody and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1. Polypeptide sequence selection and preparation of polypeptides of anti-Och1 monoclonal antibodies
[0058] Selection of Peptide Sequences
[0059] Och1 has 385 amino acids. According to its amino acid sequence information, Abimate Biomedicine (Shanghai) Co., Ltd. was entrusted to design, screen and synthesize the peptide sequence for peptide synthesis: DWKLFTGMEQ
Embodiment 2
[0060] Example 2. Preparation and purification of monoclonal antibodies against Och1
[0061] Entrusted Abimat Biopharmaceutical (Shanghai) Co., Ltd. to carry out the preparation and purification of monoclonal antibody.
[0062] 1. Antigen Production
[0063] The whole gene synthesis of the polypeptide, the gene sequence refers to the Candida Genome Database. Construction of expression vectors; identification of positive clones; plasmid extraction of positive clones and transformation of expression bacteria; large-scale induced expression production; Ni column affinity purification; after purification, protein antigens were obtained for immunization.
[0064] 2. Preparation and purification of monoclonal antibodies
[0065] Three healthy female BALB / c mice aged 8-12 weeks were immunized with the successfully obtained antigen mixture. The spleens of three mice were mixed and fused by rapid immunization of mice.
[0066] The splenocytes of the mice with the best immune respons...
Embodiment 3
[0069] Example 3. Identification and detection application of monoclonal antibodies against Och1
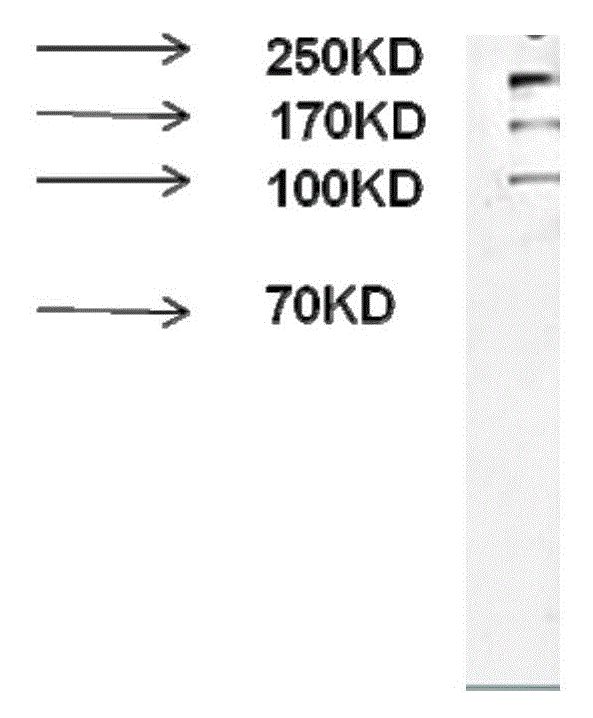
[0070] 1. Western Blot detection application of monoclonal antibody
[0071] The denatured Candida albicans cell wall protein samples were subjected to SDS-PAGE protein electrophoresis; after the electrophoresis, the semi-dry transfer was performed. Add blocking solution and block at room temperature for at least 1 h; wash the membrane 5 times with PBST washing solution, use monoclonal antibody as the primary antibody, and incubate overnight at 4°C; +L) 1:10000 dilution and incubation at room temperature for 2 hours, with slight shaking during incubation; the membrane was washed 5 times with PBST washing solution, and the NC membrane was scanned with an Odyssey infrared imaging system. see results figure 1 . The results showed that the monoclonal antibody could specifically recognize the cell wall protein of Candida albicans.
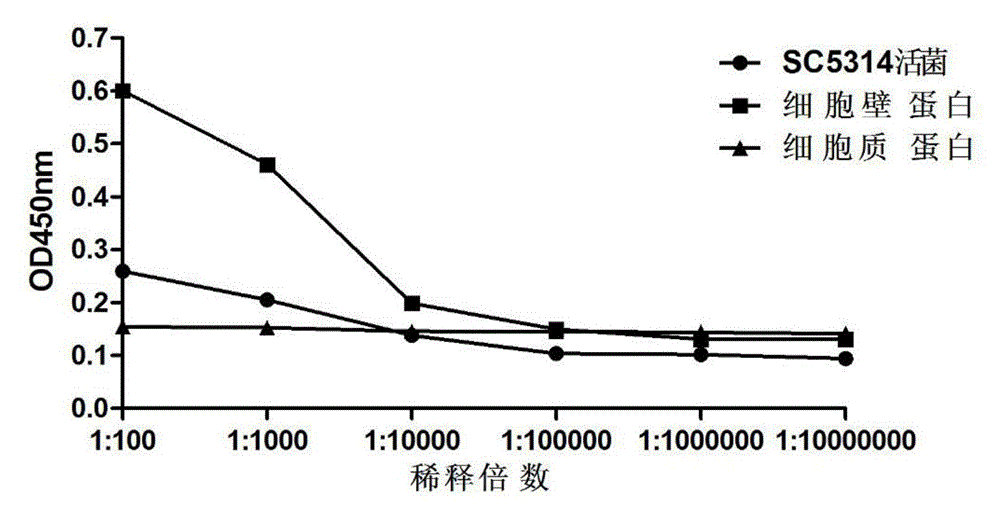
[0072] 2 Immunofluorescence detection applicatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com