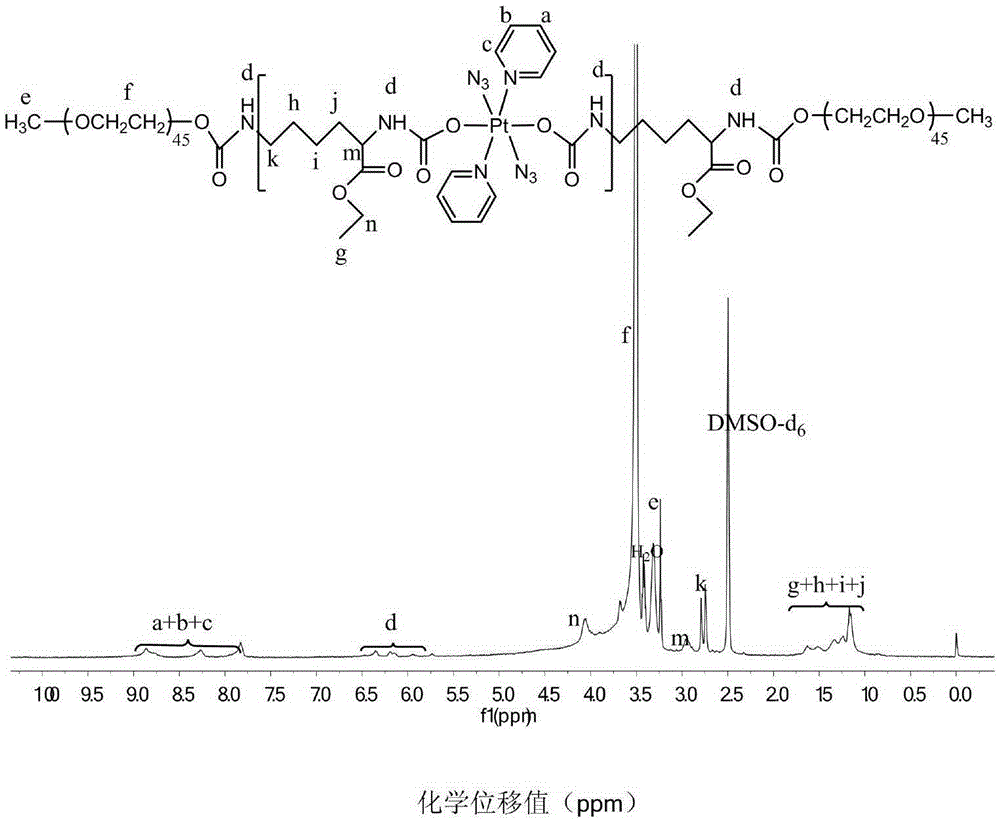
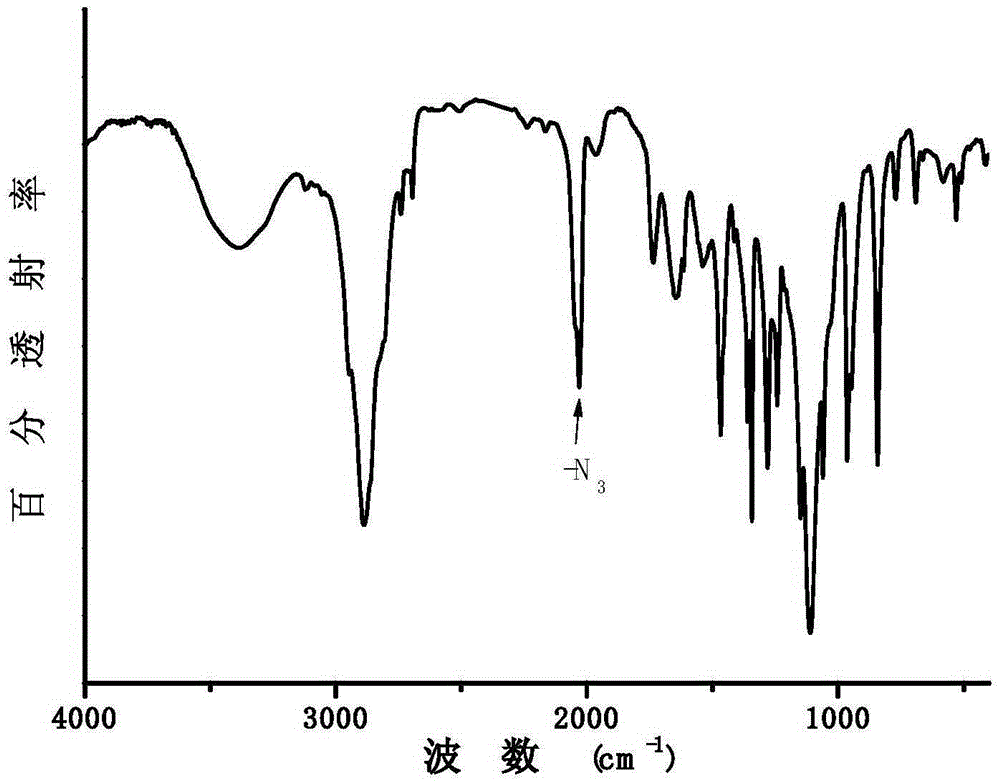
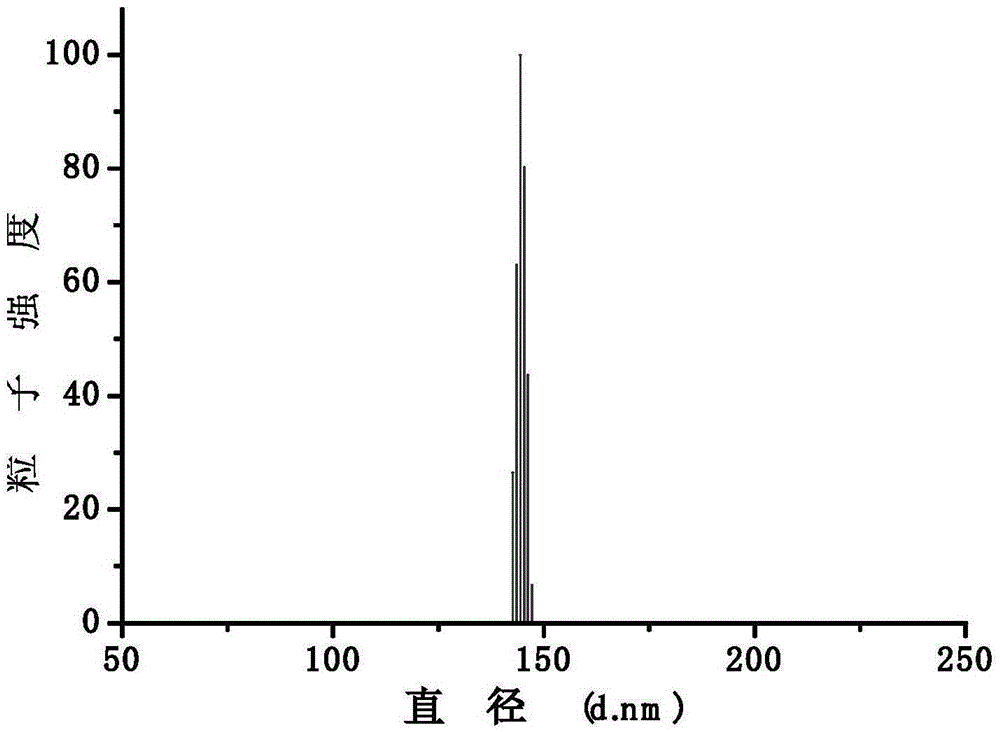
Amphipathic polymer with main chain containing photosensitive prodrug, as well as preparation method and nano-micelle of amphipathic polymer
An amphiphilic polymer and photosensitive technology, applied in the field of amphiphilic polymers containing photosensitive prodrugs in the main chain, preparation, and nanomicelle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The present invention also provides a method for preparing amphiphilic polymers containing photosensitive prodrugs in the main chain, including the following steps:
[0043] Step 1: preparing a photoactive tetravalent platinum complex;
[0044] Step 2: reacting the photoactive tetravalent platinum complex obtained in step 1 with diisocyanate to obtain the first intermediate;
[0045] Step 3: React the first intermediate obtained in Step 2 with monomethyl ether polyethylene glycol to obtain an amphiphilic polymer containing a photosensitive prodrug in the main chain.
[0046] According to the present invention, the photoactive tetravalent platinum complex is first prepared, and the preparation method preferably includes:
[0047] ① put K 2 PtCl 4 React with the ligand to obtain the ligand compound;
[0048] ② The ligand compound obtained in ① and AgNO 3 , NaN 3 reaction, and then add H 2 o 2 reaction to obtain photoactive tetravalent platinum complexes.
[0049]...
Embodiment 1
[0063] Preparation K 2 PtCl 4 (400mg, 0.96mmol) in water, K 2 PtCl 4 The concentration is 10mg / mL, react with pyridine (303.74mg, 3.84mmol) at 75°C for 24h to generate pyridine compound; then add AgNO 3 (326.4mg, 1.92mmol), reacted at 60°C for 4h, then added 1.92mmolNaN 3 , at room temperature, stirring the reaction time 24h, then adding 4.8mmolH 2 o 2. , react at room temperature for 20 min, and purify the obtained product to obtain a photoactive tetravalent platinum complex.
[0064]
Embodiment 2
[0066] Preparation K 2 PtCl 4 (500mg, 1.2mmol) in water, K 2 PtCl 4 The concentration is 10mg / mL, react with pyridine (379.69mg, 4.8mg) at 75°C for 24h to generate pyridine compound; then add AgNO 3 (408mg, 2.4mmol), reacted at 60°C for 4h, then added 6mmolNaN 3 , at room temperature, stirring the reaction time 24h, then adding 6mmolH 2 o 2. , react at room temperature for 30 min, and purify the obtained product to obtain a photoactive tetravalent platinum complex.
[0067]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com