Purification salt conversion method of micafungin
A micafungin and salt conversion technology, which is applied in the field of medicinal chemistry, can solve the problems of easy hydrolysis, unfavorable product quality control, and unfavorable industrial scale-up production, etc., and achieve the effect of ensuring product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: Preparation of 4-(5-(4-(pentyloxy)phenyl)isoxazol-3-yl)benzoic acid-1-benzotriazole ester
[0058]
[0059] Add 4-(5-(4-(pentyloxy)phenyl) isoxazol-3-yl) benzoic acid-1-benzoic acid 0.61kg, DMF5L, THF5L, HOBt0.35kg and EDC.HCl0.61kg, react at room temperature for 3-3.5 hours. After the reaction, 20 L of ethyl acetate and 5 L of purified water were added, filtered and dried to obtain 680 g of the white target product.
Embodiment 2
[0060] Embodiment 2: the preparation of compound II
[0061]
[0062] Add FR1796421.00kg (calculated as anhydrous matter) and DMF10L into a 30L glass reactor, stir and dissolve, and then add DIPEA (N,N-diisopropylethylamine) 0.21kg. Cool the reaction solution to 0-20°C, add the intermediate ① obtained in the previous step, and control the reaction temperature to 0-20°C.
[0063] After the reaction was completed, the reaction solution was poured into 100L of ethyl acetate, and a white solid was precipitated. The white solid was obtained by filtration and dried in vacuo to obtain 1500 g of Compound II.
Embodiment 3
[0064] Embodiment 3: conversion salt purification
[0065]
[0066] Dissolve 500g of compound II in purified water, apply the sample, and elute under the chromatographic conditions in the table below to collect the target components.
[0067]
[0068]
[0069] The prepared solution was collected and concentrated to dryness under reduced pressure to obtain the white product micafungin sodium, which was dried to obtain 235 g.
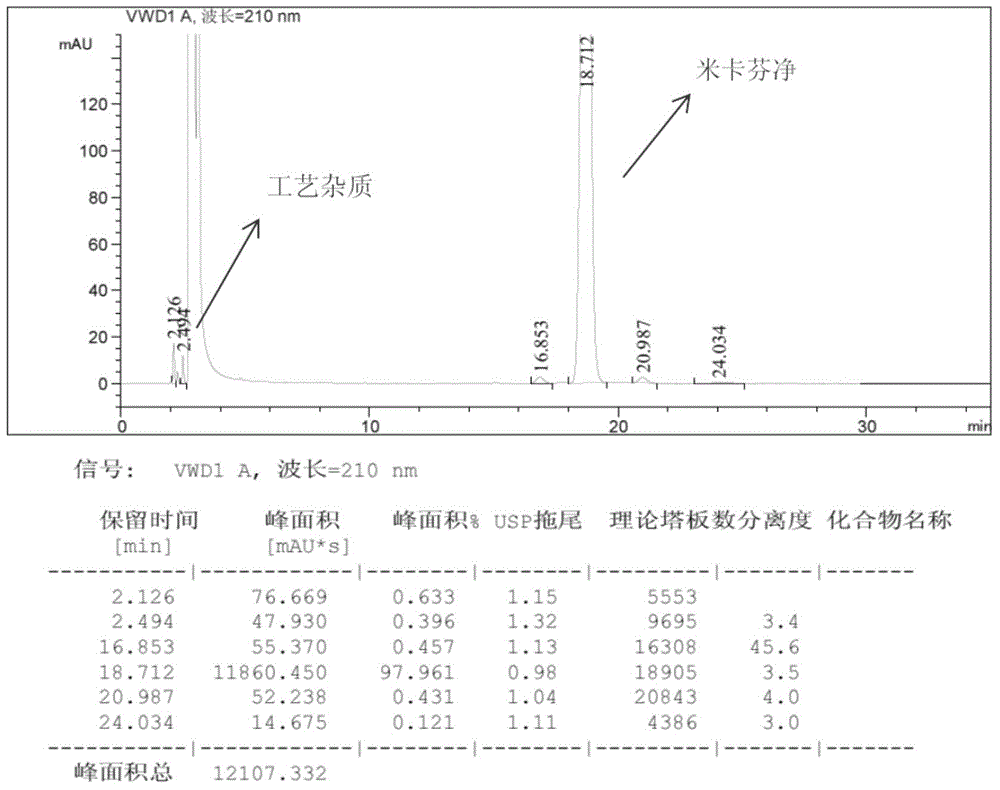
[0070] Gained sample carries out RP-HPLC detection, and purity: 99.1%, and its pattern is as follows image 3 shown.
[0071] Residue on ignition of the sample obtained: 5.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com