KPC (Klebsiella pneumoniae Carbapenemase) inhibition peptide and application thereof
A carbapenemase and inhibitory peptide technology, which is applied in the field of KPC carbapenemase inhibitory peptides, can solve the problem that the inhibitory effect of β-lactamase is not obvious, and achieve good KPC carbapenemase inhibitory activity, The effect of low production cost and good development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1KPC carbapenemase inhibitory peptide
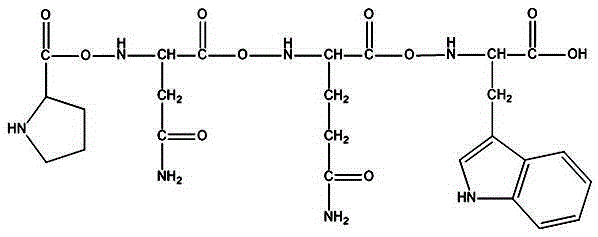
[0033] The amino acid sequence of the KPC carbapenemase inhibitory peptide is Pro-Asn-Gln-Trp, and the chemical structure is as follows figure 1 shown.
[0034] KPC carbapenemase inhibitory peptide was synthesized by solid-phase synthesis with 9-fluorenylmethoxycarbonyl (FMOC) as the N-terminal protecting group. Cleavage it from MBHA resin with a mixture of 92% trifluoroacetic acid, 5% water and 3% triisopropylsilane (TIA) (92%, 5%, and 3% are all mass percentages). After multiple precipitations of diethyl ether, it was purified by preparative reverse-phase high-performance liquid chromatography (RP-HPLC). Use C18 reverse-phase preparative column (20mm * 250mm, 5 μm); Mobile phase: 0.06% trifluoroacetic acid, 0%-80% (0.06%, 0%-80% are volume percentage) acetonitrile is mobile phase, 1.5mL / min Gradient elution was performed at a flow rate. RP-HPLC detection showed that its purity was >98%, and it w...
Embodiment 2
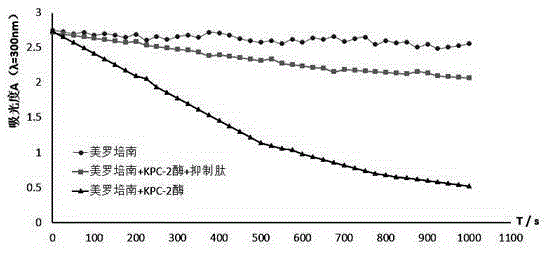
[0035] Example 2 KPC Carbapenemase Inhibiting Peptide Determination of KPC-2 Carbapenemase Inhibitory Activity
[0036] With meropenem as the reporting substrate, the experiment was divided into 3 groups, and the temperature was kept at 37±1°C:
[0037] (1) Meropenem + KPC-2 carbapenemase group: Add 5 μL of 2 mmol / L meropenem and 90 μL of 30 mmol / L HEPES buffer (pH 7.4) to a mixed solution of 5 μL 1 μmol / L of KPC-2 carbapenemase in a total volume of 100 μL.
[0038] (2) Meropenem + KPC-2 carbapenemase + enzyme inhibitory peptide group: add 5 μL of 2 mmol / L meropenem and 80 μL of 30 mmol / L HEPES buffer (pH 7.4) mixed solution Add 5 μL of KPC-2 carbapenemase at a concentration of 1 μmol / L and 10 μL of KPC carbapenemase inhibitory peptide at a concentration of 1 μmol / L for a total volume of 100 μL.
[0039] (3) Meropenem group: 95 μL of 30 mmol / L HEPES buffer (pH 7.4) was added to 5 μL of 2 mmol / L meropenem, with a total volume of 100 μL.
[0040] Since the absorbance of the r...
Embodiment 3
[0041] Example 3 KPC Carbapenemase Inhibiting Peptide and β-lactam Antibiotic Synergistic Antibacterial Action Determination
[0042] In order to carry out synergistic antibacterial experiments safely, the relatively safe genetically engineered strain E.coliRosetta(DE3) / pET28a-KPC-2 was used as a drug-resistant strain, and the E.coliRosetta(DE3) strain was used as a negative control. The minimum inhibitory concentration (Minimum Inhibitory Concentration, MIC) is determined by the double dilution method, and the synergistic antibacterial effect of the KPC carbapenemase inhibitory peptide of the present invention and β-lactam antibiotics is reflected by the change of the MIC value.
[0043] The specific method is as follows:
[0044] The engineered bacterial strain E.coliRosetta (DE3) / pET28a-KPC-2 (drug-resistant engineered bacteria) and the control strain E.coliRosetta (DE3) preserved in an ultra-low temperature refrigerator at -80°C can express KPC-2 carbapenemase They were r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com