Application of ruthenium complex serving as nucleic acid vector of target cell nucleus
A technology of ruthenium complex and nucleic acid carrier is applied in the new application field of ruthenium complex as nucleic acid carrier, and can solve the problems of gene carrier application of ruthenium complex not being reported to target cell nucleus, large molecular weight of ruthenium complex and limited effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
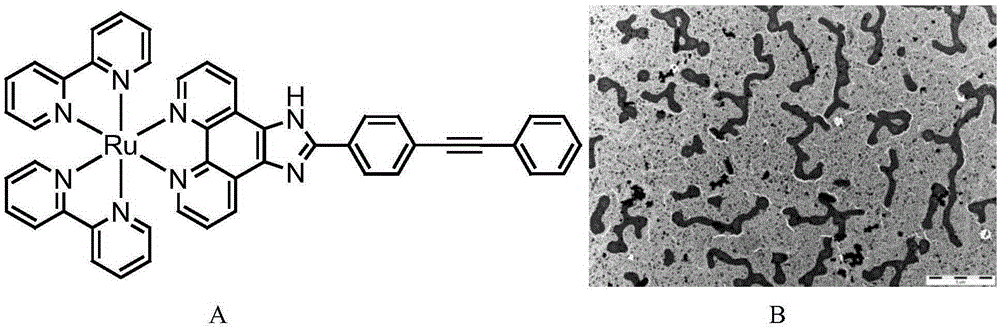
[0041] Example 1:[Ru(bpy)pBEPIP](ClO 4 ) 2 - c-myc booting section dna Preparation of complex
[0042] Experimental method: 1mM [Ru(bpy) 2 pBEPIP] (ClO 4 ) 2 ( figure 1 A) Mix evenly with 1mMc-mycPu22 solution at a ratio of 1:1, heat to 90°C for 0.5-10min, then naturally cool to room temperature, and stand at 4°C for 24 hours. The reaction mixture was dialyzed with distilled water to remove the polypyridine ruthenium (II) complex that was not loaded into the nucleic acid, the polypyridine ruthenium (II) complex-nucleic acid complex, and TEM detection found that the ruthenium complex promoted the c-mycPu22 sequence self-assembled into nanotubes ( figure 1 B).
Embodiment 2
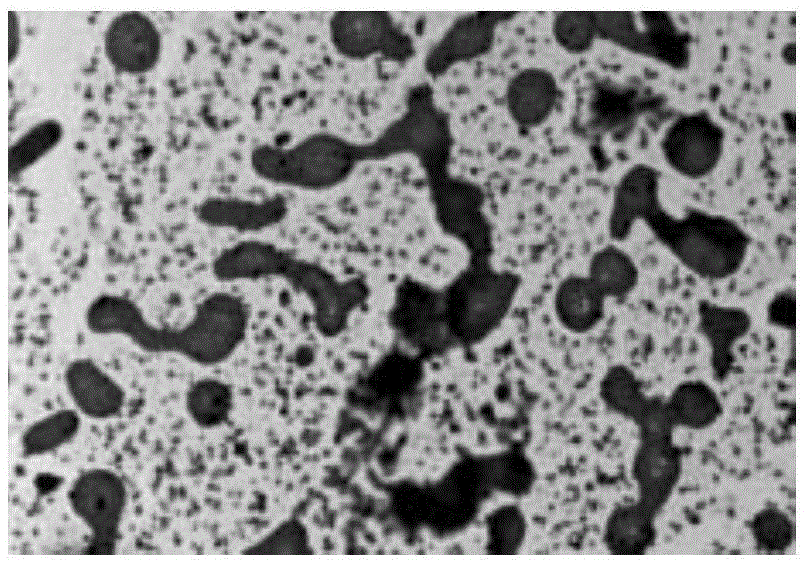
[0043] Example 2:[Ru(bpy) 2 pBEPIP] (ClO 4 ) 2 - telomere dna Preparation of complex
[0044] Experimental method: 1mM [Ru(bpy) 2 pBEPIP] (ClO 4 ) 2 Mix evenly with 1mM telomere DNA (5'-TTAGGG-3') solution at a ratio of 1:1, heat to 90°C for 0.5-10min, then naturally cool to room temperature and let stand at 4°C 24 hours. The reaction mixture was dialyzed with distilled water to remove the polypyridine ruthenium (II) complex that was not loaded into the nucleic acid, the polypyridine ruthenium (II) complex-nucleic acid complex, and TEM detection found that the ruthenium complex promoted the telomeric DNA sequence self-assembled into a nanotube-like structure ( figure 2 ).
Embodiment 3
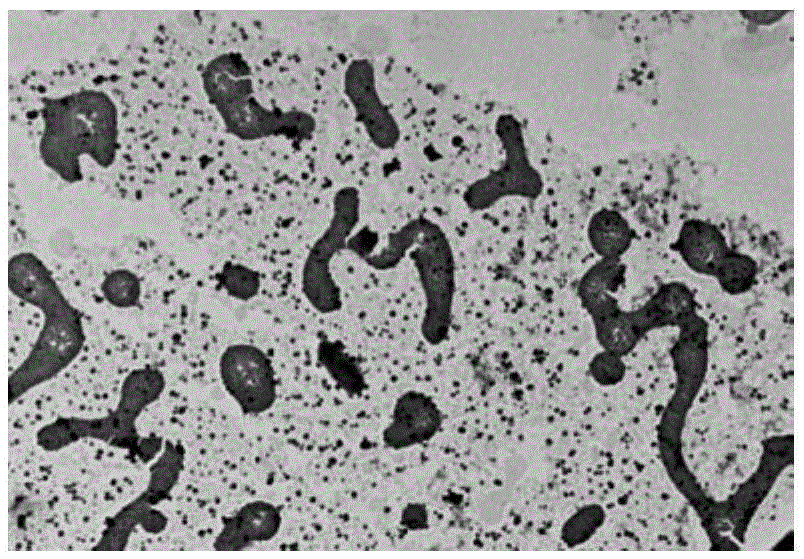
[0045] Example 3:[Ru(bpy) 2 pBEPIP] (ClO 4 ) 2 and bcl-2 booting section dna Preparation of complex
[0046] Experimental method: 1mM [Ru(bpy) 2 pBEPIP] (ClO 4 ) 2 Mix evenly with 1mMBcl-2pu27 solution at a ratio of 1:1, heat to 90°C for 0.5-10min, then naturally cool to room temperature, and stand at 4°C for 24 hours. The reaction mixture was dialyzed with distilled water to remove the polypyridine ruthenium (II) complex that was not loaded into the nucleic acid, the polypyridine ruthenium (II) complex-nucleic acid complex, and TEM detection found that the ruthenium complex promoted the telomeric DNA sequence self-assembled into a nanotube-like structure ( image 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com