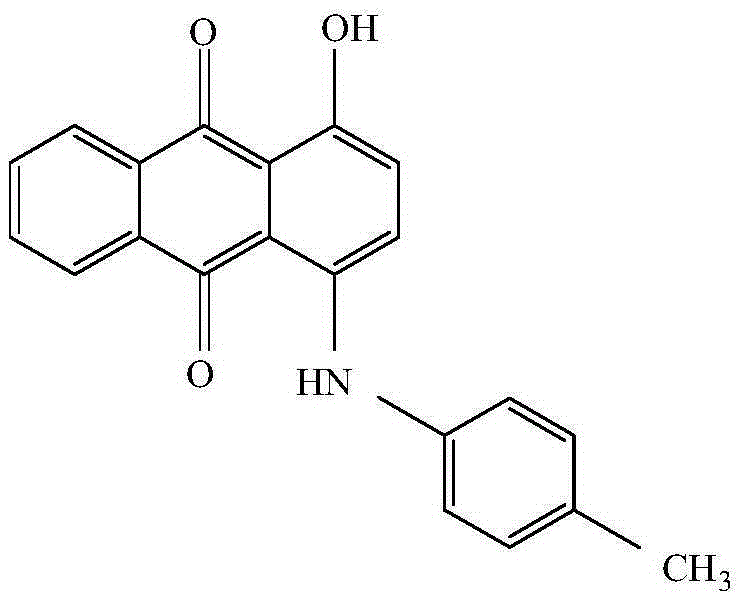
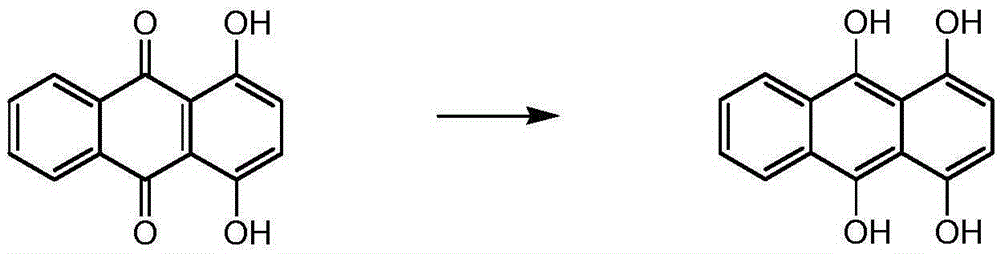
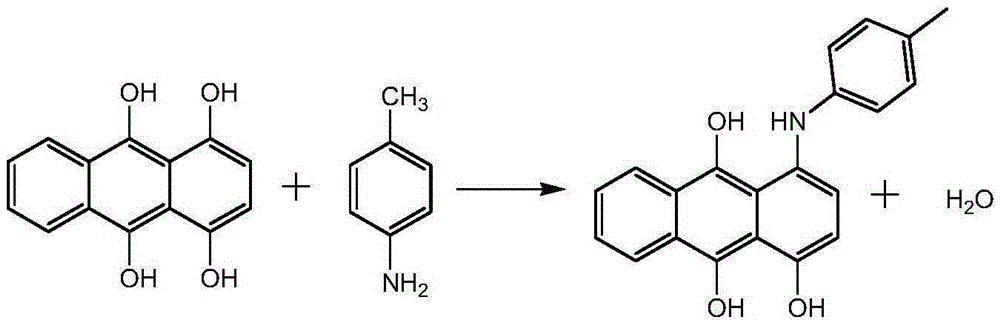
Synthetic method for solvent violet 13
A synthesis method and technology of solvent violet, applied in the field of synthesis of solvent violet 13, can solve problems such as endangering the health of operators, low reaction conversion rate, and polluting the working environment, so as to achieve increased yield, save manpower and material resources, and save waste The effect of the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] A kind of synthetic method of solvent violet 13, concrete steps are as follows,
[0049] First put methanol, 1,4-dihydroxyanthraquinone, p-nitrotoluene, iron powder, and boric acid into a pressure vessel in sequence, seal the vessel and replace it with nitrogen, then stir and raise the temperature to 50-60°C, and then inject hydrogen for hydrogenation Reduction, when the reaction system stops absorbing hydrogen, control the heat preservation reaction at 60-70°C for more than 2 hours, heat up after the reaction, and carry out the condensation reaction at 90-100°C for more than 5 hours. pressure, lower the temperature, pass air to carry out the oxidation reaction, and finally add hydrochloric acid for beating, the beating temperature is 60-70°C, filter, wash, and dry to obtain Solvent Violet 13.
[0050] Wherein, the mass ratio of 1,4-dihydroxyanthraquinone to methanol is 1:(1.1~3.0); the mass ratio of 1,4-dihydroxyanthraquinone to p-nitrotoluene is 1:(0.5~0.7); 1 , The ...
Embodiment 1
[0052] In a 500mL autoclave with mechanical stirring and a thermometer, add 44g of methanol, 40g of 1,4-dihydroxyanthraquinone, 20g of p-nitrotoluene, 1.6g of iron powder, and 5.9g of boric acid, and seal the autoclave for nitrogen replacement. , and then stirred and raised the temperature to 50°C and began to feed hydrogen for hydrogenation reduction, and the temperature was controlled at 50°C. After the reaction system stopped absorbing hydrogen, it was controlled at 70°C for heat preservation reaction to strengthen hydrogen absorption, and the heat preservation reaction time was 2 hours. After the reaction was completed, the temperature was raised to 90° C. for condensation reaction, and the reaction time was 10 h. After the reaction, the temperature was lowered to 60°C, the pressure was released, and the air was introduced to oxidize the intermediate rapidly. Finally, 8g of hydrochloric acid was added for beating. 97%, yield 87%, DC 3.0, DH 2.5, intensity 100.42.
Embodiment 2
[0054]In a 500mL autoclave with mechanical stirring and a thermometer, add 120g of methanol, 40g of 1,4-dihydroxyanthraquinone, 28g of p-nitrotoluene, 4.8g of iron powder, and 12g of boric acid, and seal the autoclave for nitrogen replacement. Then stir and raise the temperature to 55°C and start feeding hydrogen to carry out hydrogenation reduction, and the temperature is controlled at 55°C. After the reaction system stopped absorbing hydrogen, it was controlled at 60°C for heat preservation reaction to strengthen hydrogen absorption, and the heat preservation reaction time was 4 hours. After the reaction was completed, the temperature was raised to 95° C. for condensation reaction, and the reaction time was 8 hours. After the reaction, the temperature was lowered to 60°C, the pressure was released, and the air was introduced to oxidize the intermediate rapidly. Finally, 32g of hydrochloric acid was added for beating. %, the yield was 88.7%, the DC was 2.0, the DH was 1.7, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com