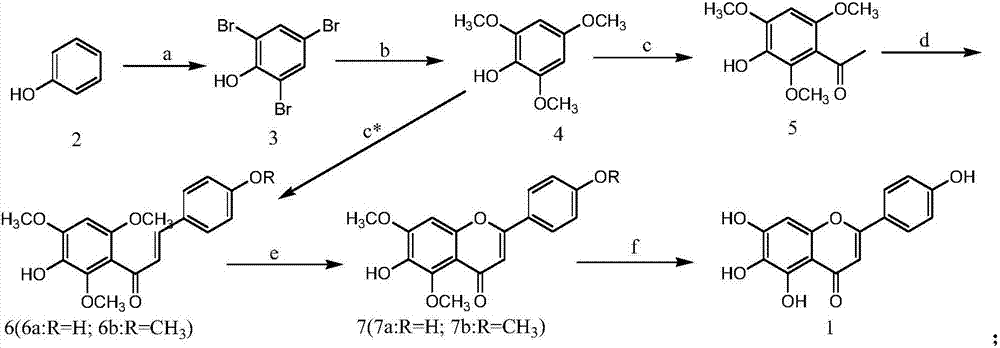
A kind of method for preparing scutellarin aglycon
A technology for scutellarin aglycone and a compound, which is applied in the field of preparing scutellarin aglycone, can solve the problems of difficult industrial application, cumbersome process operation, difficult to obtain in large quantities, etc., and achieves high industrial application value and economic value, safe operation, Easy to produce controlled effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: The preparation method of this scutellarin aglycon is as follows:
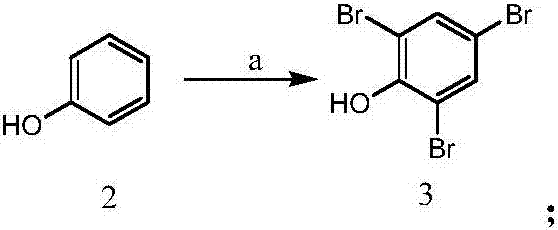
[0034] (1) The synthesis of compound 3: take by weighing 23.5g (0.25mol) phenol and place in 1000ml round bottom flask, add 300ml methyl alcohol and 130ml commercially available mass percent concentration and be 47% hydrobromic acid, then slowly under stirring at room temperature 90ml of hydrogen peroxide with a concentration of 30% by mass was added dropwise thereto, and the rate of addition was controlled so that the temperature of the reaction solution did not exceed 40°C, and the addition was completed in about 1-2 hours. After addition, continue stirring for 1-2 hours, add a little sodium bisulfite, filter after a few minutes, wash the filter cake with a small amount of methanol, press dry, and dry at 70-80°C to obtain a white powdery solid, namely compound 3; Weighing: 82.0 g, yield: 98%. 1 HNMR (DMSO): δ9.92(s, 1H), δ7.46(s, 2H).
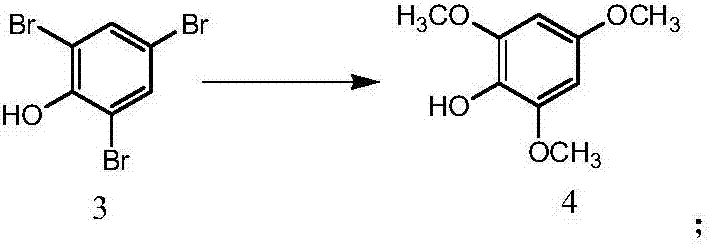
[0035] (2) Synthesis of Compound 4: Weigh 84.0g (...
Embodiment 2
[0043] Embodiment 2: The preparation method of this scutellarin aglycon is as follows:
[0044] (1) (1) Synthesis of compound 3: Weigh 23.5g (0.25mol) phenol and place it in a 1000ml round bottom flask, add 500ml ethanol, 80g (0.78mol) sodium bromide and 20ml (0.40mol) commercially available 98% Then, under vigorous stirring at room temperature, slowly add 90ml of hydrogen peroxide with a mass percentage concentration of 30% to it, and control the dropping rate so that the temperature of the reaction solution does not exceed 45°C, and the addition is completed in about 1-2 hours; after the addition, continue After stirring for 1-2 hours, add a little sodium bisulfite, filter after a few minutes, wash the filter cake with a small amount of distilled water, press dry, and dry at 70-80°C to obtain a white powdery solid, namely compound 3. Weighing: 82.0 g, yield: 99%. 1 HNMR (DMSO): the same as the previous embodiment 1 step (1).
[0045] (2) Synthesis of Compound 4: Weigh 84.0g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com