Preparation method of tyrosine enzyme inhibitor Foretinib
A tyrosinase and inhibitor technology, which is applied in the field of preparation of the compound Foretinib, can solve the problems of harsh reaction conditions, long reaction routes, and large environmental pollution, and achieves mild reaction conditions, avoiding column chromatography, and high product purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
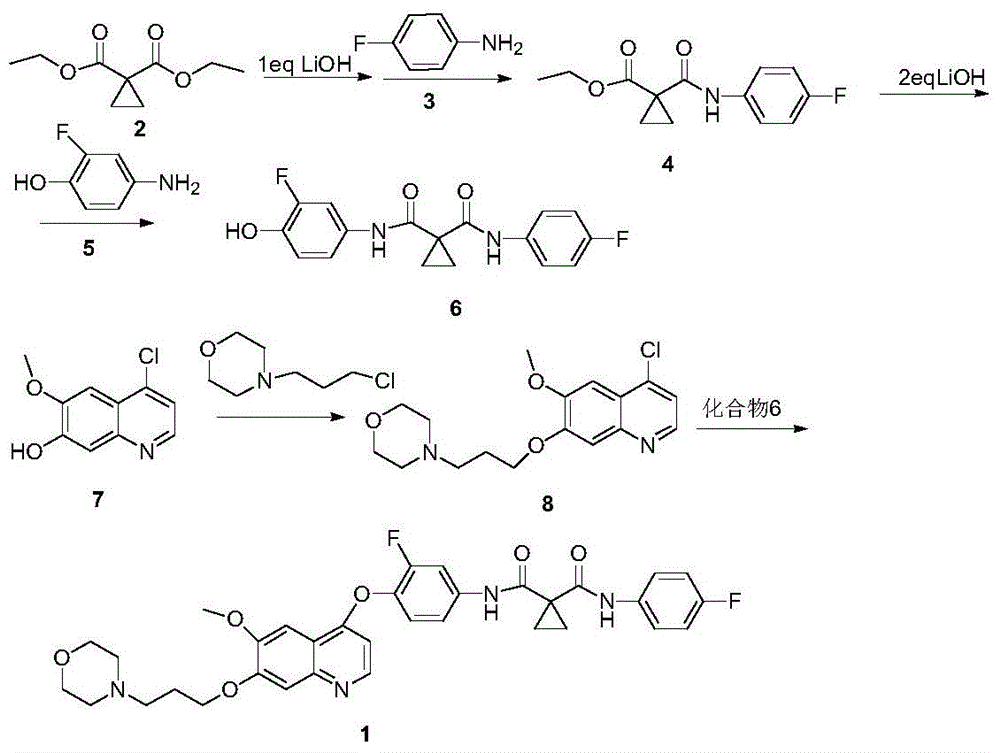
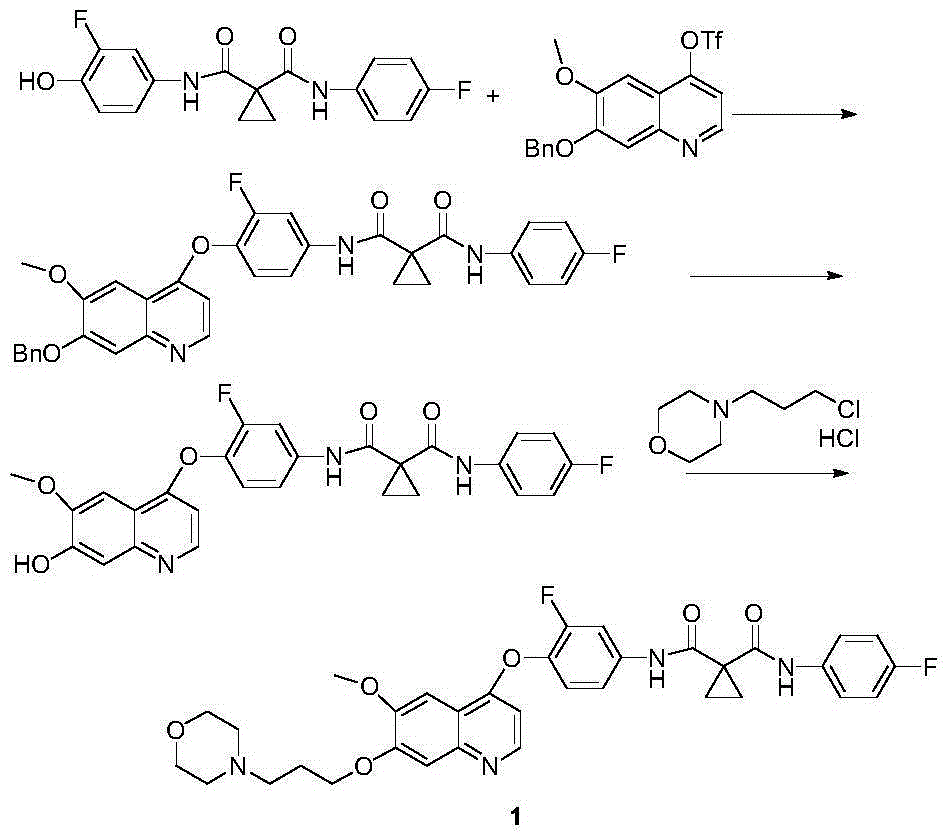
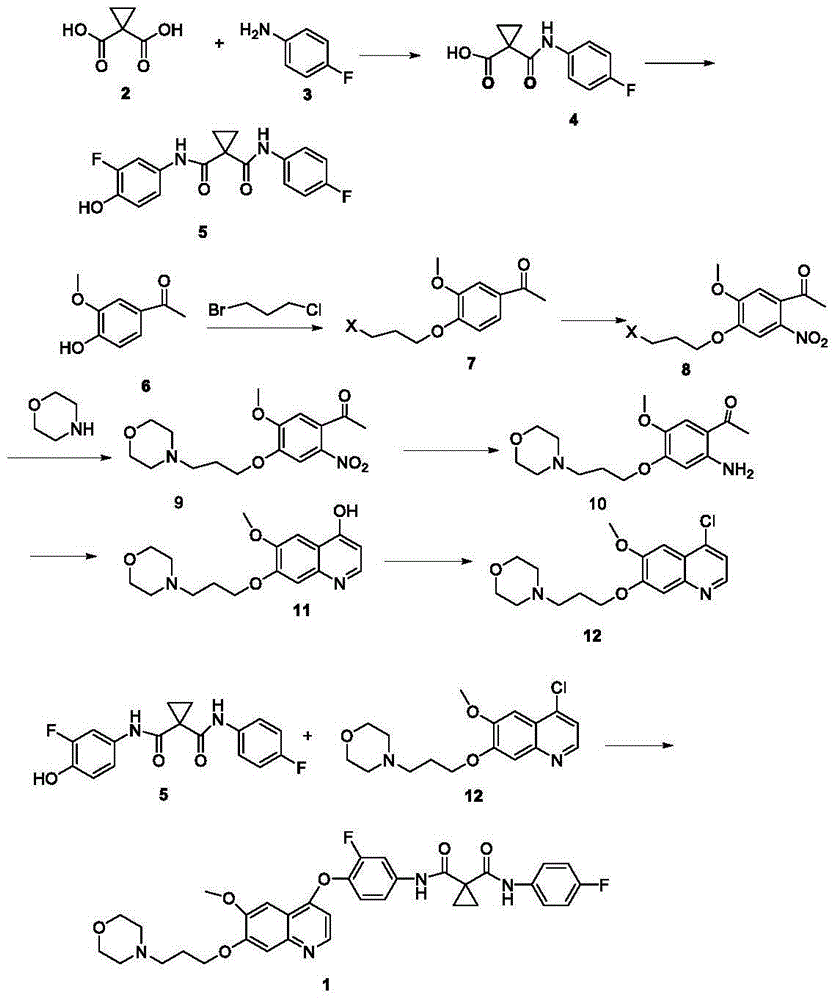
[0045] Synthesis of Compound 4
[0046] Add 372.4g (2.0mol) diethyl 1,1-cyclopropyldicarboxylate to a 5L reaction flask, add 1400mL ethanol, cool the system to 10°C in an ice-salt bath, then slowly add 62.4g (2.6mol) hydrogen dropwise A solution made of lithium oxide and 480mL of water was dropped, and kept at 35°C for 48 hours to stop the reaction. Evaporate the solvent under reduced pressure, add 1400mL water to the system, adjust the pH to about 4 with 5%-10% dilute hydrochloric acid after cooling down to 10°C, extract the system with 3X1000mL ethyl acetate, combine the organic layers, dry the organic layers, and filter , and the solvent was distilled off to obtain 311 g of a pale yellow oil. Put the product directly into a 5L reaction bottle, add 1500mL tetrahydrofuran, cool the system to 0°C, add 300g (2.4mol) oxalyl chloride and 5gDMF dropwise to the system, after the drop, react below 15°C for 3-5h and then detect the raw materials by TLC The response is complete. Th...
Embodiment 2
[0058] Synthesis of Compound 4
[0059] Add 372.4g (2.0mol) diethyl 1,1-cyclopropyldicarboxylate to a 5L reaction flask, add 1200mL isopropanol, cool the system to 10°C in an ice-salt bath, and slowly add 80.0g (2.0mol) ) sodium hydroxide and 453mL of water, after dropping, keep warm at 35°C for 30h and then stop the reaction. Evaporate the solvent under reduced pressure, add 1000mL water to the system, adjust the pH to about 4 with 5%-10% dilute hydrochloric acid after cooling down to 10°C, extract the system with 3X1000mL ethyl acetate, combine the organic layers, dry the organic layers, and filter , and the solvent was distilled off to obtain 315.7 g of a pale yellow oil. Put the product directly into a 5L reaction flask, add 1500mL tetrahydrofuran, cool the system down to 0°C, add 295.6g (2.4mol) thionyl chloride and 5gDMF dropwise into the system, after the drop is complete, react at a temperature below 15°C for 3-5h TLC detects that the reaction of raw materials is com...
Embodiment 3
[0067] Synthesis of Compound 4
[0068] Add 372.4g (2.0mol) diethyl 1,1-cyclopropyldicarboxylate to a 5L reaction flask, add 800mL 1,4-dioxane, cool the system to 10°C in an ice-salt bath, and slowly add 112.0 g (2.0mol) of potassium hydroxide and 500mL of water, after dropping, keep warm at 30°C for 24h and then stop the reaction. Add 800mL of water to the system, lower the temperature to 10°C, adjust the pH to about 4 with 5%-10% dilute hydrochloric acid, extract the system with 3X800mL ethyl acetate, combine the organic layers, dry the organic layers, filter, and evaporate the solvent to obtain 314.4g Pale yellow oil. Put the product directly into a 5L reaction flask, add 1500mL tetrahydrofuran, cool the system down to 0°C, add 295.6g (2.4mol) thionyl chloride and 5gDMF dropwise into the system, after the drop is complete, react at a temperature below 15°C for 3-5h TLC detects that the reaction of raw materials is complete. The temperature of the system was lowered to 0°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com