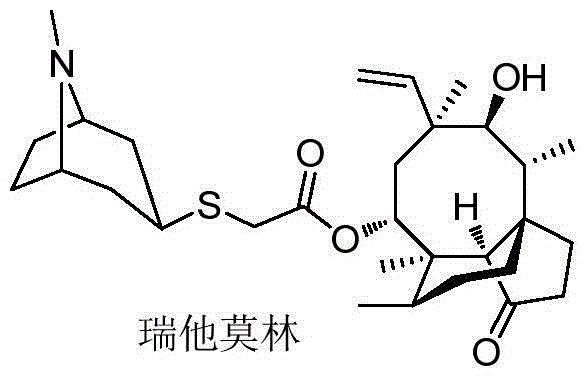
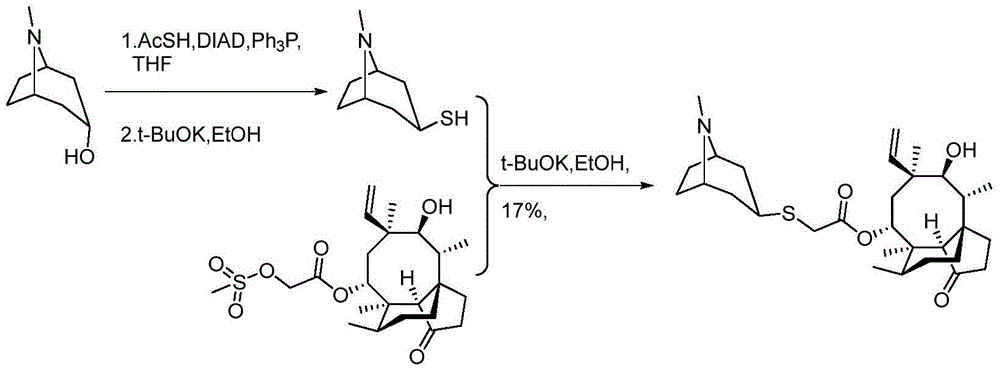
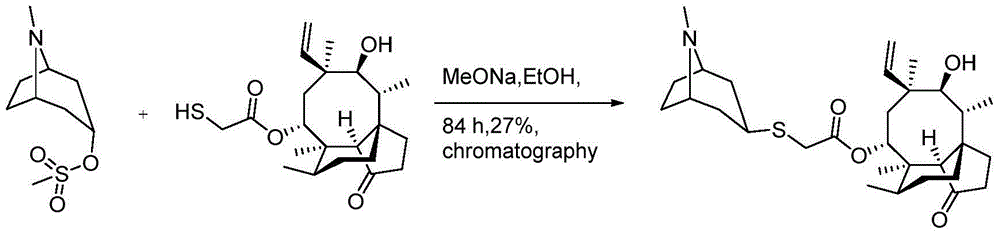
Method for preparing retapamulin
A technology of retapamulin and compounds, which is applied in the field of chemical drug synthesis, can solve problems such as flammability, low yield, and potential safety hazards, and achieve the effects of easy purification, reduced environmental impact, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Preparation of (1R,3s,5S)-8-methyl-8-azabicyclo[3.2.1]oct-3-yldiethylaminodithiocarbamate
[0053] Add endo-8-methyl-8-azabicyclo[3.2.1]octane-3-methanesulfonate (20g, 91.2mmol) and EtOH (200ml) to a 500ml four-necked flask with a thermometer and a condenser tube, After stirring and dissolving, N,N-diethyl dithiosodium trihydrate (30.82 g, 136.8 mmol) and water (20 ml) were added, and the temperature was raised to 50° C. to react for 2 h. The ethanol was evaporated under reduced pressure (external temperature 40°C), water (80ml) and ethyl acetate (80ml) were added, mixed, separated and separated, and the aqueous phase was extracted with ethyl acetate (60ml). The organic phases were combined, washed with 1mol / L NaOH (20ml), washed with water (20ml), and washed with 6mol / L HCl (30ml+30ml+15ml). The hydrochloric acid layers were combined, adjusted to pH 6-7 (75ml) with 6mol / L NaOH under an ice-water bath, a large amount of solid was precipitated, filtered with suction, t...
Embodiment 2
[0055] Preparation of (1R,3s,5S)-8-methyl-8-azabicyclo[3.2.1]oct-3-yldiethylaminodithiocarbamate
[0056] 25ml single-neck bottle was added with internal-8-methyl-8-azabicyclo[3.2.1]octane-3-methanesulfonate (1g, 4.6mmol), H 2 O (10ml), stir to dissolve, add N,N-diethyldithiocarbamate sodium trihydrate (1.54g, 6.84mmol), and react at 25°C for 10h. Add ethyl acetate (15ml+15ml) for extraction. The organic phases were combined and washed with 6mol / L HCl (5ml+3ml+3ml). The acid water layers were combined and adjusted to pH ≈ 6 with 6 mol / L NaOH under an ice-water bath. The solid was precipitated, filtered with suction, and the filter cake was washed with water, and dried at 60°C for 8 hours to obtain 0.44 g of an off-white solid with a yield of 35%.
Embodiment 3
[0058] Preparation of (1R,3s,5S)-8-methyl-8-azabicyclo[3.2.1]oct-3-yldiethylaminodithiocarbamate
[0059] To a 500ml four-necked flask with a thermometer and a condenser tube, add endo-8-methyl-8-azabicyclo[3.2.1]octane-3-methanesulfonate (20g, 91.2mmol), DMF (100ml), After stirring and dissolving, sodium N,N-diethyldithiocarbamate trihydrate (30.82 g, 136.8 mmol) was added, and the temperature was raised to 120° C. to react for 1 h. DMF was evaporated under reduced pressure (external temperature 70°C), water (80ml) and ethyl acetate (80ml) were added, mixed, separated and separated, and the aqueous phase was extracted with ethyl acetate (60ml). The organic phases were combined, washed with 1mol / L NaOH (20ml), washed with water (20ml), and washed with 6mol / L HCl (30ml+30ml+15ml). The hydrochloric acid layers were combined, and the pH was adjusted to 6-7 with 6 mol / L NaOH under an ice-water bath to precipitate a large amount of solid, which was filtered with suction, washed w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com