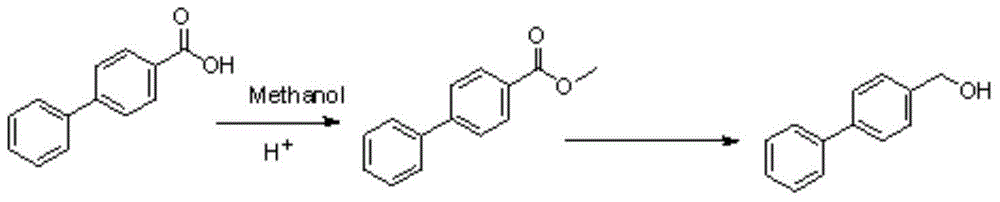
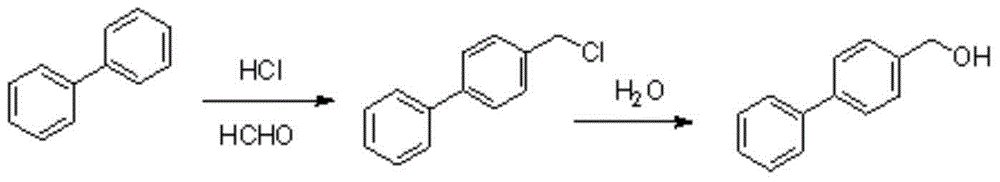
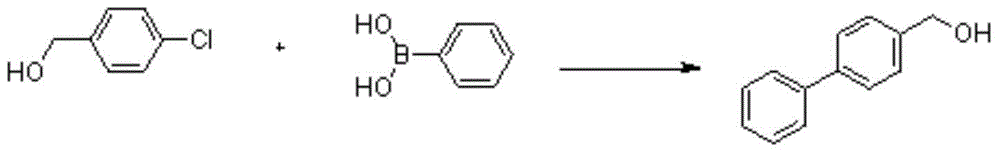4-biphenyl methanol synthetic method
A biphenylmethanol and synthesis method technology, applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., can solve the problems of high production raw material cost, unsuitable for scale-up production, etc., and achieve low price and easy operation , The effect of easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Into a 3L autoclave, add biphenyl (350g, 2.27mol), cuprous chloride (1.0g, 0.01mol), paraformaldehyde (90g, 1.0mol), 85% phosphoric acid (270g) and concentrated hydrochloric acid (1050g) ), the reaction system is closed, the pressure in the kettle is about 3 atmospheres, slowly heated and stirred to 120°C, reacted for 36 hours, HPLC detects the reaction, cools down, down to 30°C, stands still, removes the lower water layer, and adds to the reaction kettle Water (1400g), start stirring, heat up, heat to 100°C and reflux, react for 24 hours, then cool down to room temperature, filter to obtain a crude product, recrystallize with three times the amount of toluene to obtain 217 grams of white solid, and pass the conventional The structure confirmation method was confirmed to be 4-biphenylmethanol, the purity detected by HPLC: 98%, and the yield: 52%.
[0052] Nuclear magnetic confirmation of the structure of the white solid (final product) 1 HNMR: (400MHz, CDCl3) δ: 4.73 (s, 2...
Embodiment 2
[0054] Add biphenyl (350g, 2.27mol), cuprous chloride (1.0g, 0.01mol), paraformaldehyde (90g, 1.0mol), 85% phosphoric acid (270g) and concentrated hydrochloric acid (1050g) to a 3L three-necked flask. ), a conventional normal pressure reaction system, slowly heat and stir to 120°C, react for 36 hours, HPLC check the reaction, cool down, drop to 30°C, stand still, remove the lower water layer, add water (1400g) to the reactor, and start stirring Raise the temperature, heat to 100°C and reflux, react for 24 hours, then cool down to room temperature, filter to obtain the crude product, recrystallize with three times the amount of toluene to obtain 129 grams of white solid, and by conventional structure confirmation methods, the confirmation is 4 -Biphenylmethanol, purity detected by HPLC: 98%, yield: 31%.
[0055] Nuclear magnetic confirmation of the structure of the white solid (final product) 1 HNMR: (400MHz, CDCl3) δ: 4.73 (s, 2H), 7.35 (m, 1H), 7.44 (m, 4H), 7.59 (d, 4H, J=8.0)....
Embodiment 3
[0057] Into a 3L three-neck flask, add biphenyl (350g, 2.27mol), paraformaldehyde (90g, 1.0mol), 85% phosphoric acid (270g) and concentrated hydrochloric acid (1050g), normal pressure reaction system, slowly heating and stirring to 120°C, react for 36 hours, HPLC detects the reaction, cools down, drops to 30°C, stands still, removes the lower water layer, adds water (1400g) to the reaction kettle, starts to stir and raises, heat to 100°C to reflux, and react for 24 hours. Then, it was cooled to room temperature and filtered to obtain a crude product, which was recrystallized with three times the amount of toluene to obtain 90 g of a white solid, which was confirmed to be 4-biphenylmethanol by conventional structure confirmation methods. Its purity as determined by HPLC: 98 %, yield: 22%.
[0058] Nuclear magnetic confirmation of the structure of the white solid (final product) 1 HNMR: (400MHz, CDCl3) δ: 4.73 (s, 2H), 7.35 (m, 1H), 7.44 (m, 4H), 7.59 (d, 4H, J=8.0).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com