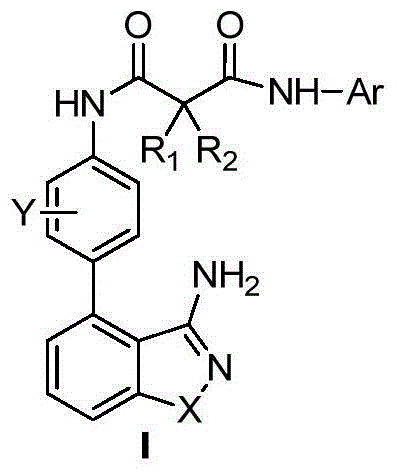
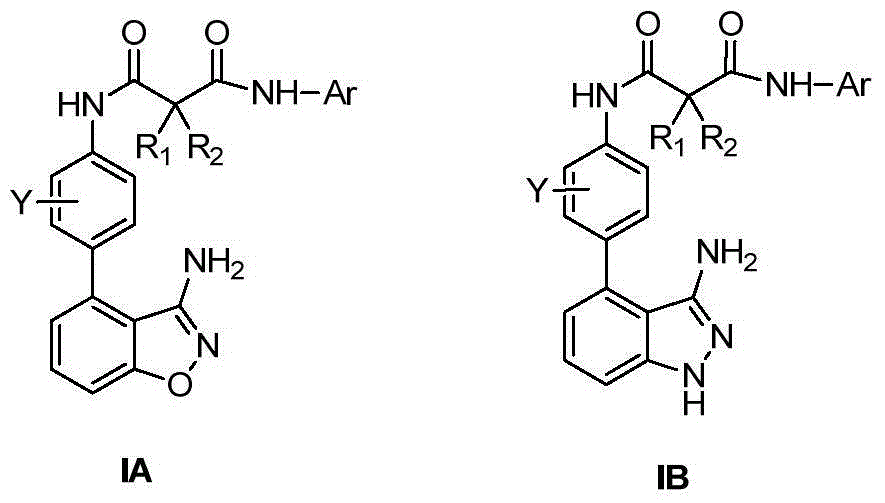
3-amino-benzo five-membered heterocyclic compounds and preparation method and applications thereof
A five-membered heterocycle and compound technology, applied in the field of 3-amino-benzo five-membered heterocycle compounds, can solve the problems of weakening the direct relationship and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] General formula for the preparation of compounds IA-1 to IA-19
[0056]
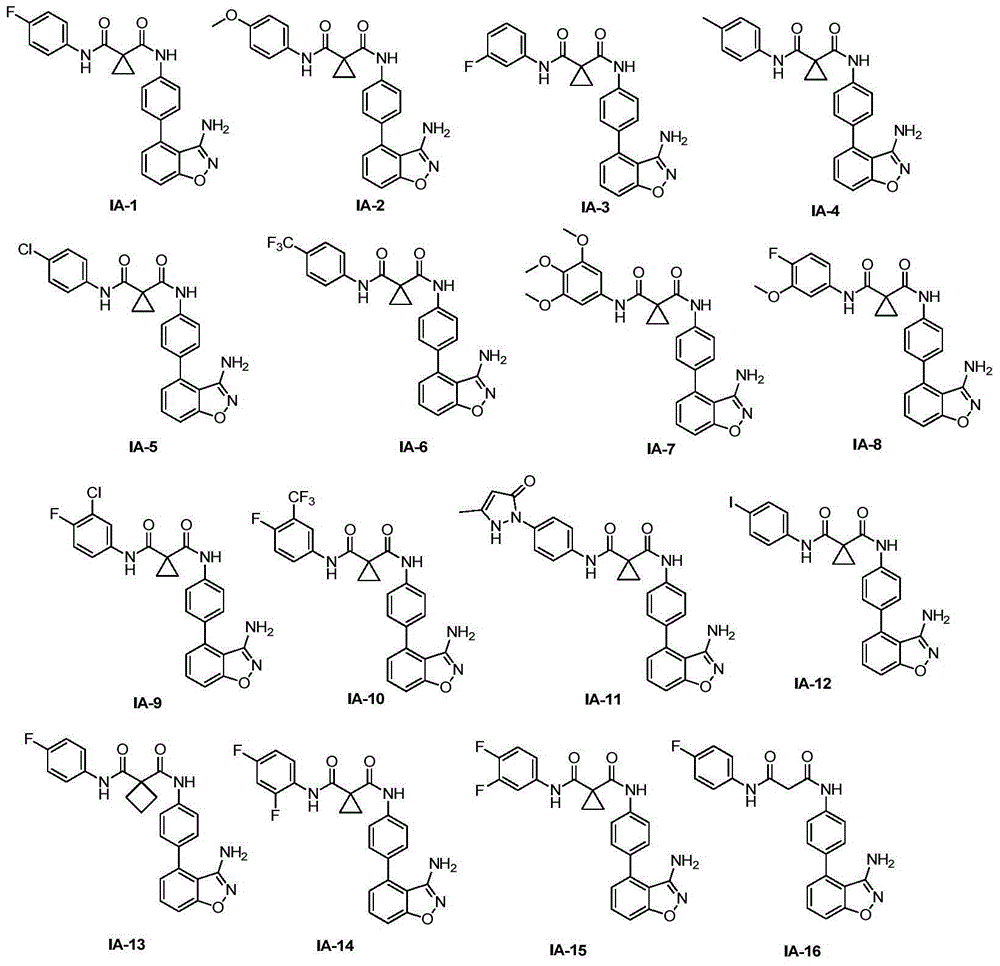
[0057] Compound 1 (its synthetic reference JiZQetal, JMedChem2008, 51:1231-1241) (1equiv) was mixed with various acids 2 (its synthetic reference WO:2005 / 030140A2) (1equiv), and an appropriate amount of dichloromethane (DCM ) or dimethyl sulfoxide (DMF), stirred at 0°C, added HATU (1.8 equiv), stirred for 5 min, then added triethylamine (TEA) (1 equiv), stirred for 5 min, raised to room temperature and stirred overnight. After the reaction was monitored by TLC, dichloromethane or ethyl acetate was added to dilute the reaction solution, the organic layer was washed 3 times with water, the organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated, and column chromatography (0-10% Methanol: dichloromethane) to give the target product IA. Table 1. Corresponding structural formulas of starting materials 2a–2s for the synthesis of compound IA
[0058...
preparation Embodiment 1
[0059] Preparation Example 1: Preparation of Compound IA-1
[0060]
[0061] Compound 1 (40 mg, 0.178 mmol) was mixed with acid 2a (40 mg, 0.178 mmol), dissolved in 3 mL of dichloromethane, stirred at 0 ° C, added HATU (122 mg, 0.32 mmol), stirred for 5 min, and then added triethylamine ( 25μL, 0.178mmol), after stirring for 5min, rise to room temperature and stir overnight. After the reaction was monitored by TLC, 40 mL of dichloromethane was added to dilute the reaction solution, the organic layer was washed with water (3 × 20 mL), the organic layer was washed with saturated brine (20 mL), dried over anhydrous sodium sulfate, filtered, concentrated, and column chromatography ( 0-30% methanol:dichloromethane) afforded the target product IA-1 as a white solid (55 mg, 72%).
[0062] 1 HNMR (300MHz, CDC1 3 )δ9.63(s,1H),8.77(s,1H),7.69(d,J=8.5Hz,2H),7.58–7.44(m,5H),7.40(d,J=8.4Hz,1H), 7.16–6.98(m,3H),4.18(s,2H),1.77–1.62(m,4H).
preparation Embodiment 2
[0063] Preparation Example 2: Preparation of Compound IA-2
[0064] IA-2 was synthesized as a white solid (74%) in the same manner as IA-1 except that the corresponding acid 2b was used.
[0065] 1 H-NMR (300MHz, CDC1 3 )δ10.11(s,1H),8.08(s,1H),7.71(d,J=8.7Hz,2H),7.55–7.34(m,6H),7.11(d,J=7.2Hz,1H), 6.89(d,J=8.9Hz,2H),4.15(s,2H),3.80(s,3H),1.79(dd,J=7.6,4.8Hz,2H),1.62–1.58(dd,J=7.6, 4.8Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com