Compound composition of obeticholic acid and berberine and applications thereof
A kind of technology of obeticholic acid and berberine hydrochloride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Drug efficacy experiment of embodiment 1 liver cirrhosis model rat
[0028] Take clean grade SD rats (body weight 180-220g) and divide them into sham operation group and model group at random. After the rats are given 10% chloral hydrate solution (300mg / kg) intraperitoneal injection anesthesia, open the abdomen along the midline of the abdomen, find And the common bile duct was exposed. In the sham operation group, only the common bile duct was separated and then the abdomen was closed. After the common bile duct was ligated at the proximal end around the hilar bile duct in the model group, the abdomen was routinely closed. Penicillin 400,000 units per mouse was injected continuously for 3 days after operation. feed.
[0029] The liver cirrhosis model rats with successful modeling were randomly divided into model group (administered only 1% methylcellulose), berberine group (administered BBR, 100mg.kg -1 .d -1 ), obeticholic acid group (administration of OCA, 10mg.kg ...
Embodiment 2
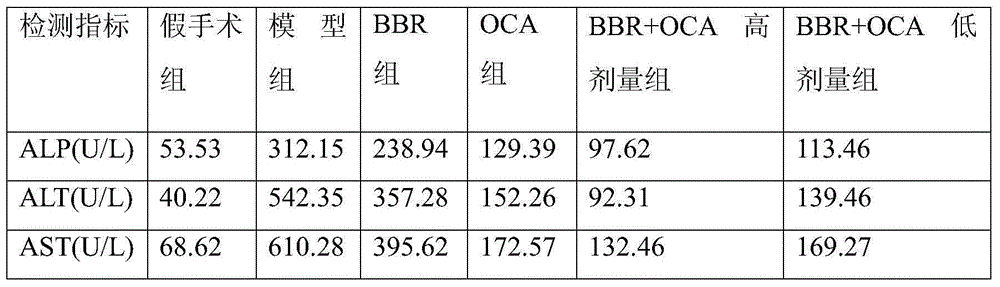
[0033] Example 2 Drug efficacy experiment on rats with abnormal liver and blood indicators caused by high-fat diet
[0034] Get SD rats and feed with basal feed for one week as the adaptation period, and then randomly divide them into 6 groups: (1) normal group (control), fed with low-fat diet; (2) high-fat diet group, fed with high-fat diet (78.85 % raw grain, 21% lard, 0.15% cholesterol) feeding; (3) BBR group, high-fat feed (same as above) feeding+berberine (gavage, 180mg / kg, qd); (4) OCA group, high-fat feed (same as above) feeding+obeticholic acid (gavage, 10mg / kg, qd); (5) OCA+BBR high-dose group, high-fat feed (same as above) feeding+obeticholic acid combined with berberine high-dose group (gavage Stomach, 10mg / kg+180mg / kg, qd); (6) OCA+BBR low-dose group, high-fat diet (same as above) feeding + obeticholic acid combined with berberine high-dose group (gastric administration, 5mg / kg+90mg / kg, qd). Correspondingly, the normal group and the high-fat diet group were intr...
Embodiment 3
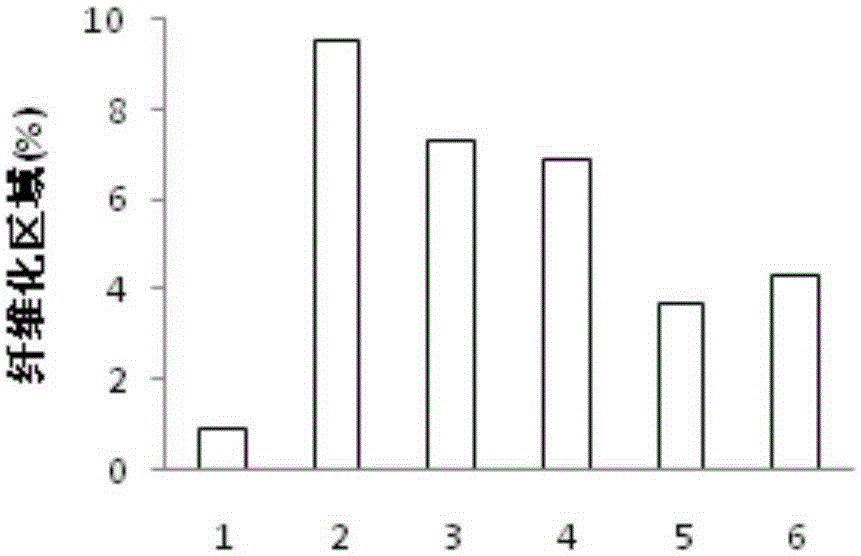
[0038] Example 3 Drug efficacy experiment on liver fibrosis model mice
[0039] After one week of adaptive feeding, male C57 / B6 mice were randomly divided into normal group (Control), CCL 4 group, BBR group (administration of BBR100mg / kg), OCA group (administration of OCA10mg / kg), OCA+BBR high-dose group (10mg / kg+100mg / kg), OCA+BBR low-dose group (5mg / kg+50mg / kg). Except the normal group, all other groups were intraperitoneally injected with 10% CCl three times a week 4 2μl / g, for 4 consecutive weeks, the normal group was intraperitoneally injected with the same amount of olive oil. Since the first day of modeling, the corresponding drugs were given by intragastric administration at the same time, and the normal group and the CCL 4 Groups were given an equal volume of 1% methylcellulose. After the experiment, blood was taken to detect liver function indicators such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST); liver was taken for liver HE patholo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com