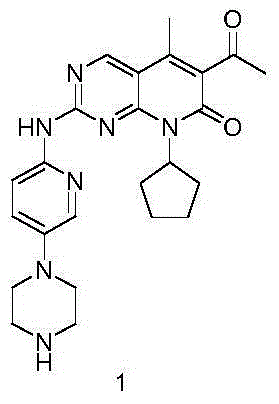
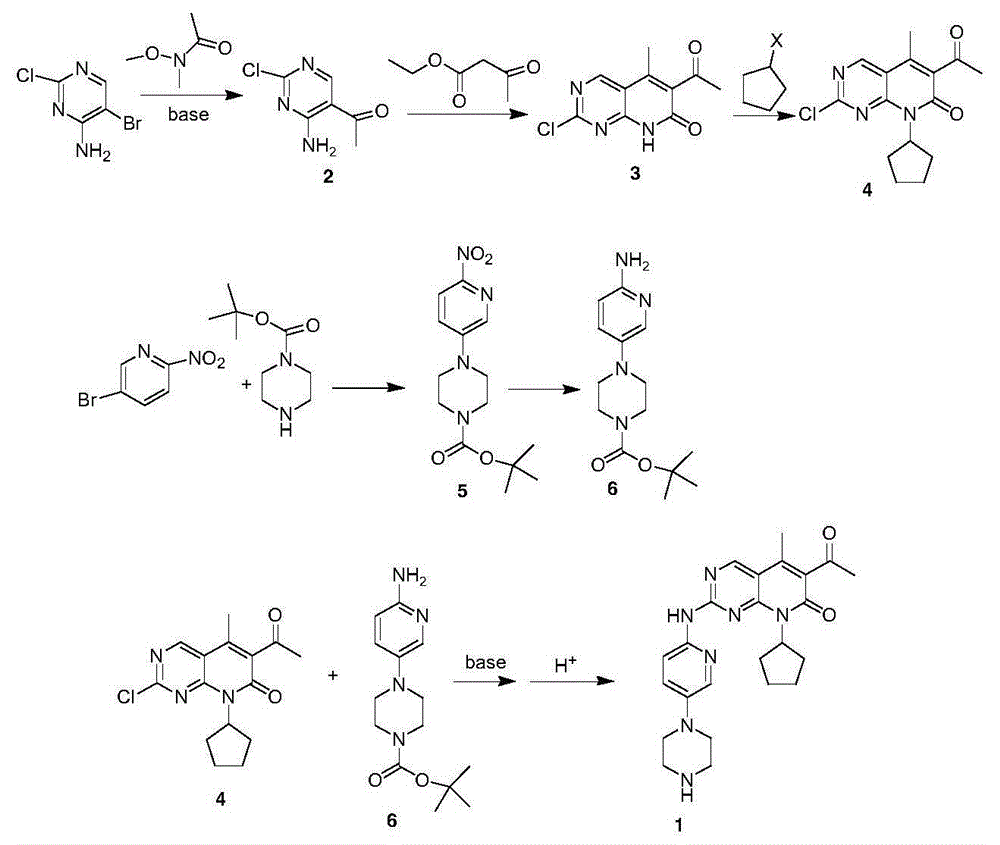
Preparation method for selective kinases inhibitor Palbociclib
The technology of a kinase inhibitor and a reducing agent is applied in the field of pharmaceutical preparation, which can solve the problems of difficulty in purchasing raw material compounds and intermediate products, and achieve the effects of low cost, simple reaction mechanism and stable yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Synthesis of Intermediate 2
[0027] Add 20.8g (0.1mol) of compound I and 208mL of tetrahydrofuran into a 1L reaction flask. After stirring to dissolve, the system cools down to -78°C. After the temperature stabilizes, add 62.5mL of n-butyllithium-n-hexane (concentration: 15%) dropwise. . After dropping, keep stirring at this temperature for 10 min, and at the same time add dropwise a solution of 12.9 g (0.125 mol) of N-methoxy-N-methylacetamide and 129 mL of tetrahydrofuran. After dropping, stir at -78°C for 1 hour, then the system naturally warms up to 0°C, add 100mL of saturated ammonium chloride solution, stir for 30min, extract with 3X300mL ethyl acetate, combine the organic layer, wash the organic layer with 3X300mL saturated salt water, and anhydrous magnesium sulfate After drying, filtering and concentrating, 15.8 g of crude yellow solid was obtained, yield: 91.9%, which was directly used in the next step.
[0028] Synthesis of intermediate 3
[0029] 10.3g (...
Embodiment 2
[0039] Synthesis of Intermediate 2
[0040] Add 20.8g (0.1mol) of compound I and 208mL tetrahydrofuran into a 1L reaction flask. After stirring to dissolve, the system cools down to -78°C. After the temperature stabilizes, 11.2g (0.1mol) of potassium tert-butoxide is added in batches. After the addition, keep stirring at this temperature for 30 min, and at the same time add dropwise a solution of 12.9 g (0.125 mol) of N-methoxy-N-methylacetamide and 129 mL of tetrahydrofuran. After dropping, stir at -78°C for 1 hour, then the system naturally warms up to -10°C, add 20mL of water dropwise, then slowly drop in 20mL of 1N hydrochloric acid, add 40mL of saturated sodium bicarbonate solution, stir for 30min, then use 3X300mL ethyl acetate Extract and combine the organic layers. The organic layer was washed with 3×300 mL saturated brine, dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain 14.5 g of yellow solid crude product, yield: 84.3%, which was directly...
Embodiment 3
[0052] Synthesis of Intermediate 2
[0053] 20.8g (0.1mol) of compound I and 208mL of tetrahydrofuran were added to a 1L reaction flask, stirred to dissolve, and then 5.4g (0.1mol) of sodium methoxide was added, and the temperature of the system was raised to 60°C. After the addition, keep stirring at this temperature for 1.5 h, and at the same time add dropwise a solution of 12.9 g (0.125 mol) of N-methoxy-N-methylacetamide and 129 mL of tetrahydrofuran. After dropping, stir at 60°C for 2 hours and monitor the system. The reaction of the raw materials is complete. Add 200mL of water and 200mL of ethyl acetate after distilling off the solvent, beat for 10min and then separate into layers. Concentrate to obtain 12.2 g of yellow solid crude product, yield: 70.9%, which is directly used in the next step.
[0054] Synthesis of intermediate 3
[0055] 10.3g (0.06mol) of intermediate 2, 8.6g (0.066mol) of ethyl acetoacetate, 16.2g (0.3mol) of sodium methoxide and 103mL of methanol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com