Method for constructing eukaryotic expression vector capable of efficiently expressing pig CYP1A1 genes
A eukaryotic expression vector, -CYP1A1 technology, applied in the field of molecular biology, can solve complex problems such as expression regulation, and achieve remarkable beneficial effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
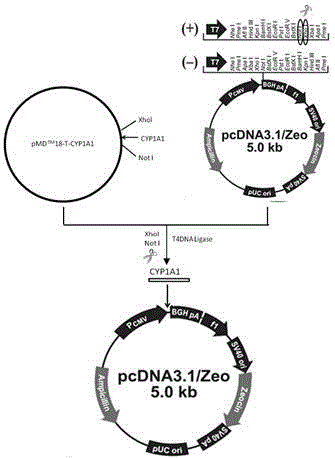
[0042] Example 1. Construction of a eukaryotic expression vector for high-efficiency expression of porcine CYP1A1 gene
[0043] 1. Cloning of porcine CYP1A1 gene
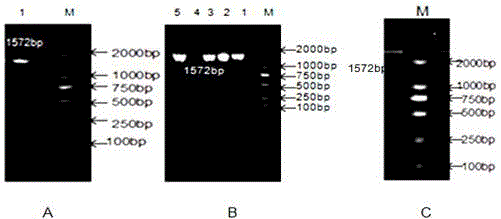
[0044] The experimental pigs were slaughtered Xiaomeishan pigs collected from the Shanghai Meishan Pig Breeding Farm to collect liver tissue samples, extract total RNA, detect RNA purity and concentration by 2.2% formaldehyde denaturing gel electrophoresis and ultraviolet spectrophotometer, and synthesize cDNA by reverse transcription. Primers were designed using the cDNA sequence of the pig CYP1A1 gene in GenBank (NCBIReferenceSequence: NM_214412.1) as a template. In order to facilitate the construction of recombinant vectors, a NotⅠ restriction site (GCGGCCGC) and four protective bases (ATTT) were added to the upstream sequence of the primer, and the downstream The sequence was added with an XhoI restriction site (CTCGAG) and three protective bases (CCG) to form specific primers. Using the reverse transcription cDNA...
Embodiment 2
[0050] Example 2. Verification of the expression effect of pcDNA3.1 / zeo(+)-CYP1A1 eukaryotic expression vector transfected into pig PAM cells
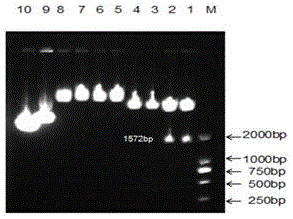
[0051] Prepare 100ug / ml, 150ug / ml, 200ug / ml, 250ug / ml, 300ug / ml Zeocin screening medium (Invitrogen, Carlsbad, CA), and culture PAM cells respectively (see literature: Meng Chunhua, Cao Shaoxian, Su Lei, etc. Isolation, purification and cryopreservation of porcine alveolar macrophages. Jiangsu Agricultural Sciences, 2012, 40(11): 198-20), select the concentration of Zeocin to completely kill PAM cells in 10 to 14 days as the screening concentration of Zeocin (250μg / ml). Normal PAM cells were inoculated in a six-well plate, cultured in complete 1640 medium containing 10% fetal bovine serum and 1% double antibody for 24 hours, and the cell adhesion was observed. When the degree of adhesion reached 80%, transfection reagent Lipofectmine TM In 2000, the pcDNA3.1 / zeo(+)-CYP1A1 plasmid and the pcDNA3.1 / zeo(+) plasmid were transfected into ...
Embodiment 3
[0054] Example 3. Mycoplasma pneumoniae infection experiment of PAM cell line overexpressing porcine CYP1A1 gene
[0055] Use 10 respectively 6 CCU / mL Mycoplasma hyopneumoniae (Mhp) bacterial liquid (see literature: Shao Guoqing, Liu Maojun, Sun Peiyuan et al. Establishment of experimental swine model of swine asthma. Microbiology and Infection, 2007, 2(4): 215-218.) infection adherent 80% CYP1A1 overexpression PAM cells (constructed by the transfection experiment in Example 2 above) (denoted as overexpression group), pcDNA3.1 / zeo (+) vector transfected cells (denoted as empty group), normal For PAM cells (denoted as normal group), 500ml of Mhp bacteria solution and 1000ml of double-antibody-free RPMI1640 culture solution per well. Cells were collected at 0h, 4h, 12h, 24h, and 48h after transfection, total cellular RNA was extracted and reverse-transcribed, and the infection effect was identified by PCR amplification of the specific membrane protein P36 gene of Mycoplasma hyo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com