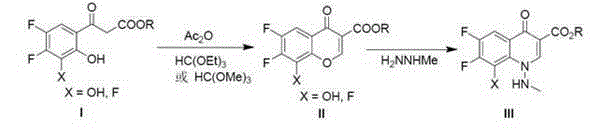
Preparation for marbofloxacin key intermediate
A technology of marbofloxacin and intermediates, applied in the field of medicinal chemistry, can solve the problems of too long reaction route, expensive chemical reagents, unfavorable use and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Preparation of 6,7,8-trifluoro-4-oxo-4-hydrogen-benzopyran-3-carboxylic acid ethyl ester (formula II, X=F, R=Et)
[0031] 3-(3,4,5-trifluoro-2-hydroxyphenyl)-3-keto-propionic acid ethyl ester (formula I, X=F, R=Et) (50g, 0.191mmol, 1.0eq), original A mixture of triethyl formate (85g, 0.574mmol, 3.0eq) and acetic anhydride (100g, 0.98mmol, 5.1eq) was heated in an oil bath until the system refluxed, and the refluxed state was kept for 24 hours. After the system cooled down, it was concentrated on a rotary evaporator, and the residue was added to ethyl acetate (1000L), and the obtained organic solution was washed twice with saturated sodium bicarbonate solution (2×200mL) and once with brine (100mL). Dry over sodium sulfate, filter out the desiccant, concentrate the obtained organic phase under reduced pressure, recrystallize the residue using a mixed solvent of ethyl acetate and heptane, and dry the product in vacuum at 60°C to constant weight to obtain a light ...
Embodiment 2
[0032] Example 2: Preparation of 6,7,8-trifluoro-4-oxo-4-hydrogen-benzopyran-3-carboxylic acid ethyl ester (formula II, X=F, R=Et)
[0033] 3-(3,4,5-trifluoro-2-hydroxyphenyl)-3-keto-propionic acid ethyl ester (formula I, X=F, R=Et) (50g, 0.191mmol, 1.0eq), original A mixture of triethyl formate (65g, 0.44mmol, 2.3eq) and acetic anhydride (78g, 0.76mmol, 4.0eq) was heated in an oil bath until the system refluxed, and the refluxed state was kept for 6 hours. After the system cooled down, it was concentrated on a rotary evaporator, and the residue was added to ethyl acetate (1000L), and the obtained organic solution was washed twice with saturated sodium bicarbonate solution (2×200mL) and once with brine (100mL). Dry over sodium sulfate, filter out the desiccant, and concentrate the obtained organic phase under reduced pressure. The residue is recrystallized with a mixed solvent of ethyl acetate and heptane, and the product is vacuum-dried at 60°C to constant weight to obtain a ...
Embodiment 3
[0034] Example 3: Preparation of 6,7-difluoro-8-hydroxyl-4-oxo-4-hydrogen-chromene-3-carboxylic acid methyl ester (formula II, X=OH, R=Me)
[0035]3-(4,5-difluoro-2,3-dihydroxyphenyl)-3-keto-propionic acid methyl ester (formula I, X=OH, R=Me) (50g, 0.20mmol, 1.0eq), A mixture of trimethyl orthoformate (51g, 0.48mmol, 2.4eq) and acetic anhydride (105g, 1.03mmol, 5.1eq) was heated in an oil bath until the system refluxed, and the refluxed state was kept for 12 hours. After the system cooled down, it was concentrated on a rotary evaporator, and the residue was added to ethyl acetate (1000L), and the obtained organic solution was washed twice with saturated sodium bicarbonate solution (2×200mL) and once with brine (100mL). Dry over sodium sulfate water, filter out the desiccant, concentrate the obtained organic phase under reduced pressure, recrystallize the residue using a mixed solvent of ethyl acetate and heptane, and dry the product in vacuum at 60°C to constant weight to obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com