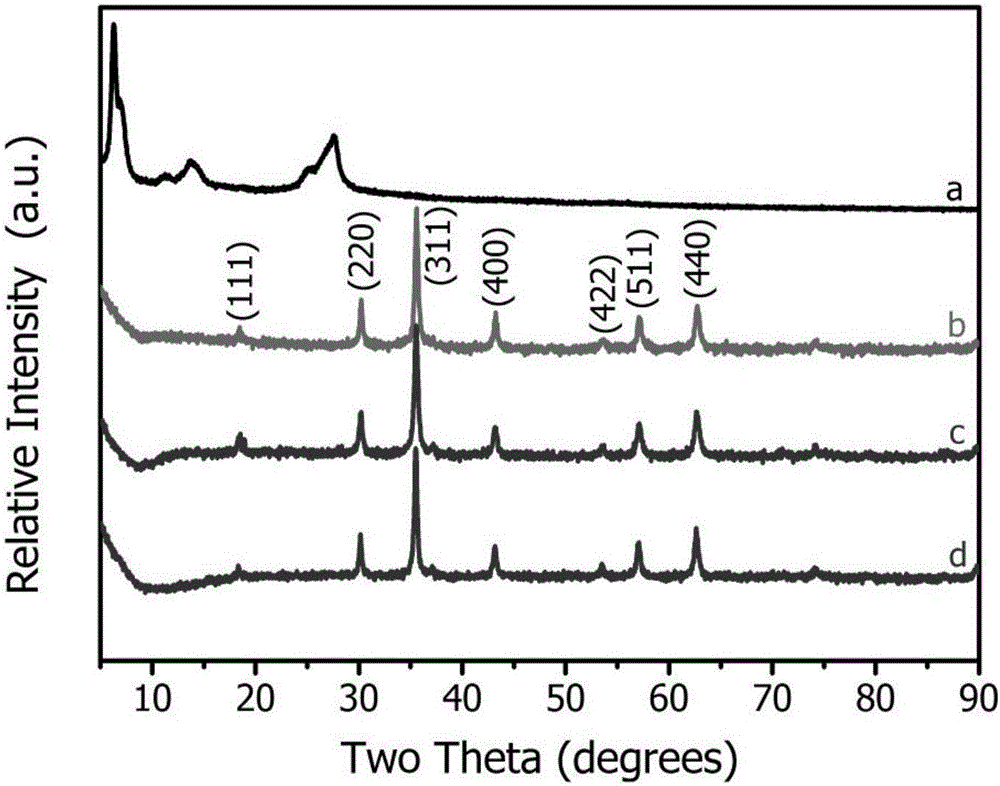
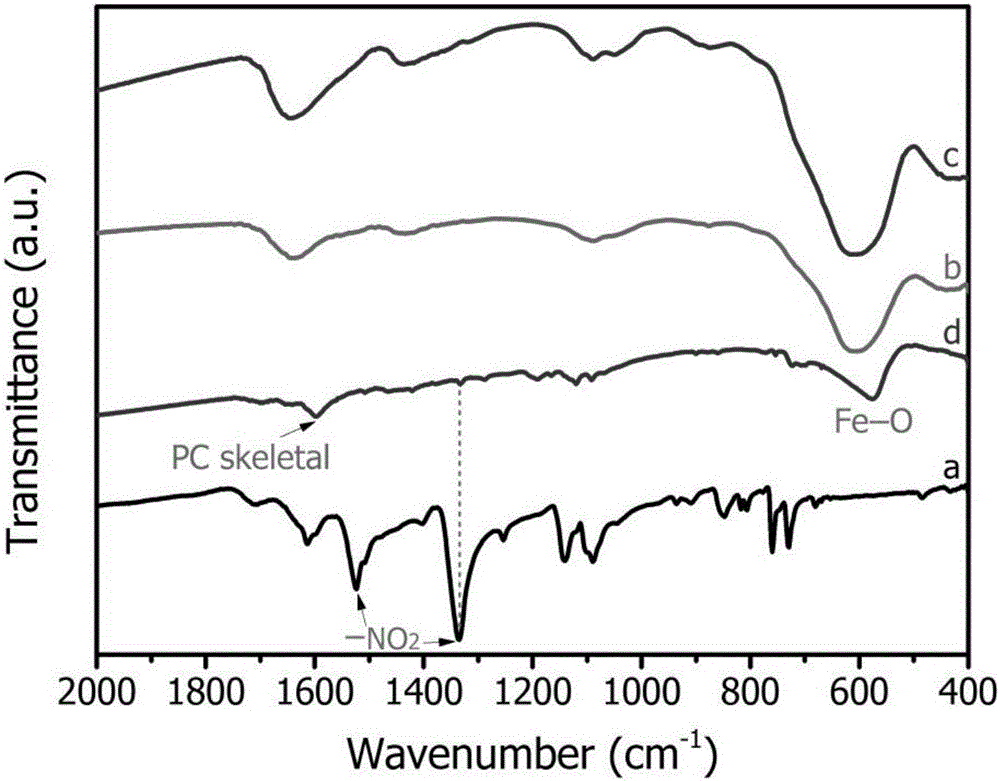
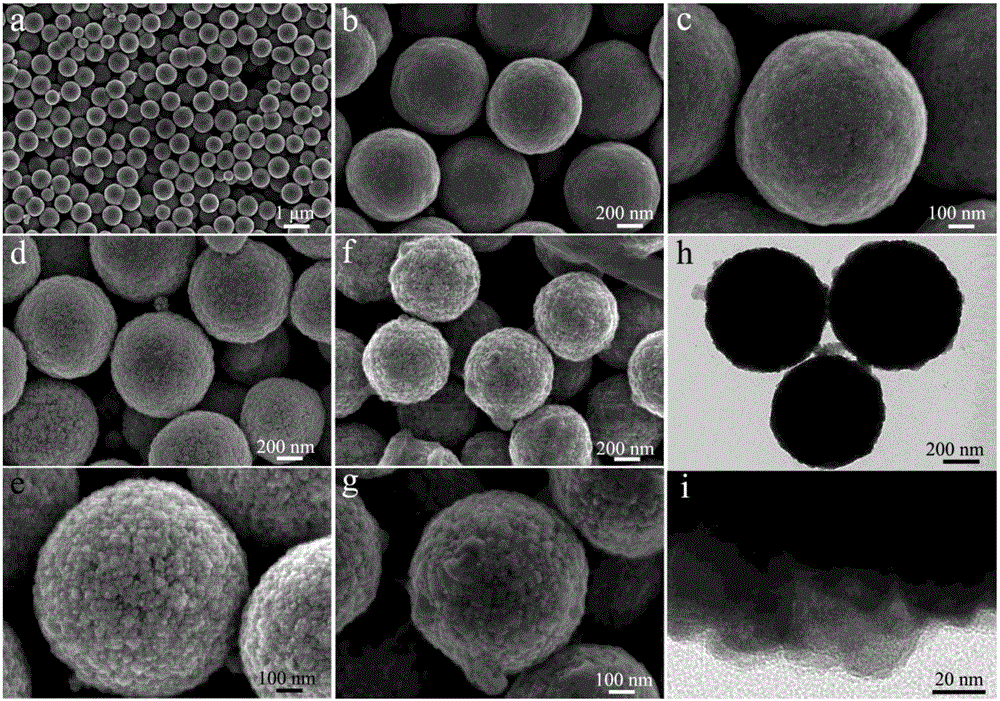
Magnetic recyclable copper tetranitrophthalocyanine composite catalyst and application thereof in phenolic pollutant chromogenic recognition reaction
A composite catalyst, tetranitro technology, applied in the direction of organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, analysis through chemical reaction of materials, etc. and other problems, to achieve the effect of low price, simple preparation, non-toxic and harmless cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Step (1) Fe 3 o 4 Preparation of magnetic carrier:
[0057] Dissolve 0.64g of sodium dodecylbenzenesulfonate (SDBS) and 3.72g of sodium acetate in 65mL of ethylene glycol, ultrasonically disperse for 15min with stirring to dissolve completely, then add 2.16g of FeCl 3 ·6H 2 After O was magnetically stirred for 30 min, the mixture was transferred into a 100 mL hydrothermal reaction kettle, and reacted at a temperature of 180 °C for 12 h. After the reaction kettle was naturally cooled to room temperature, the product was washed three times with deionized water and absolute ethanol, and the obtained samples were dried in a vacuum oven at 60°C for 12 hours and collected for use.
[0058] Step (2) Fe 3 o 4 Preparation of C:
[0059] Add 7.90g of glucose into 70mL of deionized water, stir and wait for it to dissolve completely, then take 0.46g of Fe prepared in step (1) 3 o 4 The powder was added to aqueous glucose solution for ultrasonic dispersion for 5 minutes, the...
Embodiment 2
[0072] Dissolve 0.80g of sodium dodecylbenzenesulfonate (SDBS) and 4.50g of sodium acetate in 75mL of ethylene glycol, ultrasonically disperse for 20min with stirring to dissolve completely, then add 2.50g of FeCl 3 ·6H 2 After O was magnetically stirred for 30 min, the mixture was transferred into a 100 mL hydrothermal reactor, and reacted at a temperature of 190 °C for 8 h. After the reaction kettle was naturally cooled to room temperature, the product was washed three times with deionized water and absolute ethanol, and the obtained samples were dried in a vacuum oven at 60°C for 12 hours and collected for use.
[0073] Step (2) Fe 3 o 4 Preparation of C:
[0074] Add 9.0g of glucose into 70mL of deionized water, stir until it is completely dissolved, take 0.60g of Fe prepared in step (1) 3 o 4 The powder was added to aqueous glucose solution for ultrasonic dispersion for 5 minutes, and then the reaction solution was transferred to a 100mL reactor and reacted at 190°C ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com