Antibacterial cefotiam hydrochloride drug composition
A technology of cefotiam hydrochloride and antibacterial drugs, applied in the field of medicine, can solve the problems of loss of antibacterial activity, poor mixing uniformity, unstable β-lactam ring, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Preparation of Cefotiam Hydrochloride Crystals
[0024] Prepare a saturated ethanol solution of cefotiam hydrochloride crude product at 30°C, and then add a mixed solvent of isobutanol and petroleum ether whose volume is 8 times the volume of the saturated ethanol solution, and the volume ratio of the isobutanol and petroleum ether is 2:1.5 , after stirring evenly, stir while cooling down, the cooling rate is 10°C / hour, the stirring speed is 105 rpm, and at the same time, add ether twice the volume of the mixed solvent of isobutanol and petroleum ether, and stop after cooling down to 0°C Stirring, standing still for crystal growth for 3 hours, filtering, and drying under reduced pressure to obtain cefotiam hydrochloride crystalline compound.
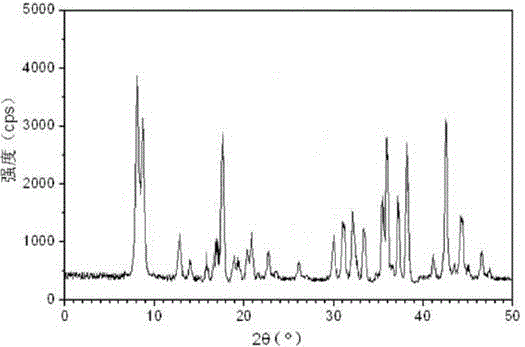
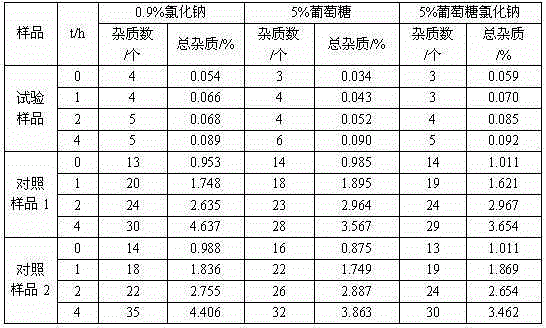
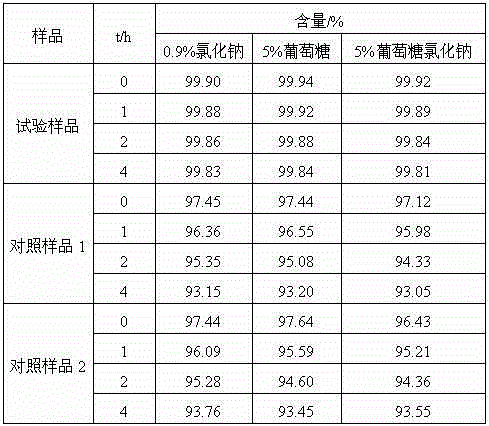
[0025] The prepared cefotiam hydrochloride crystal uses Cu-Kα ray to measure the X-ray powder diffraction pattern that obtains as follows figure 1 Shown, its purity as determined by high performance liquid chromatogr...
Embodiment 2
[0026] Example 2: Preparation of cefotiam hydrochloride composition
[0027] The composition comprises: 1 part by weight of cefotiam hydrochloride crystal prepared by the present invention, and 0.01 part by weight of arginine.
[0028] The preparation method is:
[0029] (1) Weigh cefotiam hydrochloride crystals and arginine in proportion and mix them thoroughly;
[0030] (2) Dispense into sterilized vials and stopper them.
Embodiment 3
[0031] Example 3: Preparation of cefotiam hydrochloride composition
[0032] The composition comprises: 1 part by weight of cefotiam hydrochloride crystal prepared by the present invention, and 0.02 part by weight of arginine.
[0033] The preparation method is:
[0034] (1) Weigh cefotiam hydrochloride crystals and arginine in proportion and mix them thoroughly;
[0035] (2) Dispense into sterilized vials and stopper them.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com