Method for synthesizing cyclopeptide by multicomponent reaction under enzyme catalysis actions
A multi-component reaction and cyclic peptide technology, applied in fermentation and other directions, can solve problems such as ring formation, and achieve the effects of simple process, high purity, and simple separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
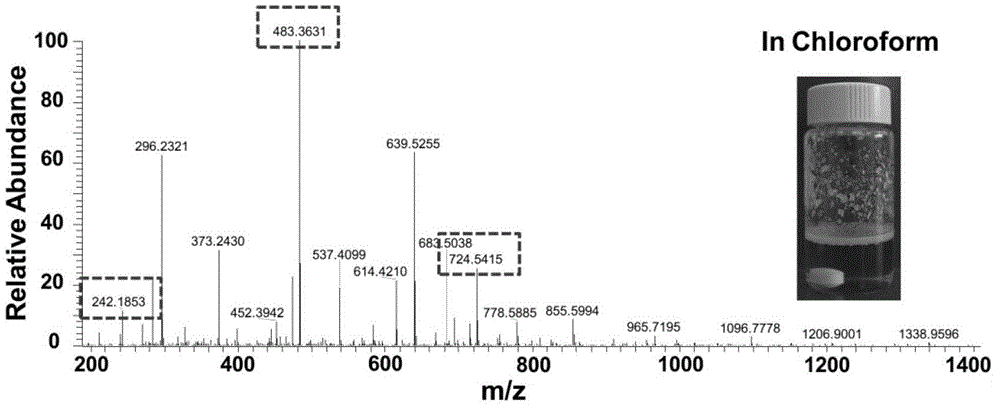
[0034] Chloroform phase: Add 5 ml of chloroform to a 20 ml vial, and ventilate argon for ten minutes to remove the oxygen in the chloroform. Then add 20% of the total mass of the reactants (diamine, isonitrile, aldehyde) of Candida antarctica lipase, 1 mmol of n-butyraldehyde and 1 mmol of 2,2-dimethyl-1 to the solution , 3-propanediamine, and stir at room temperature for 15 minutes. After the reaction solution was completely clear, 1 mmol of ethyl isonitrile was added to the reaction solution, and the reaction solution was stirred at room temperature for 48 hours. After the reaction, the reaction solution is bright yellow clear liquid, the reaction solution is separated from the lipase, and the composition of the reaction solution is directly analyzed by mass spectrometry. The chloroform phase is not purified by HPLC.
[0035] The reaction solution was characterized by mass spectrometry. Among the numerous ion signal peaks, three special signal peaks of 242.2, 483.4 and 724.5...
Embodiment 2
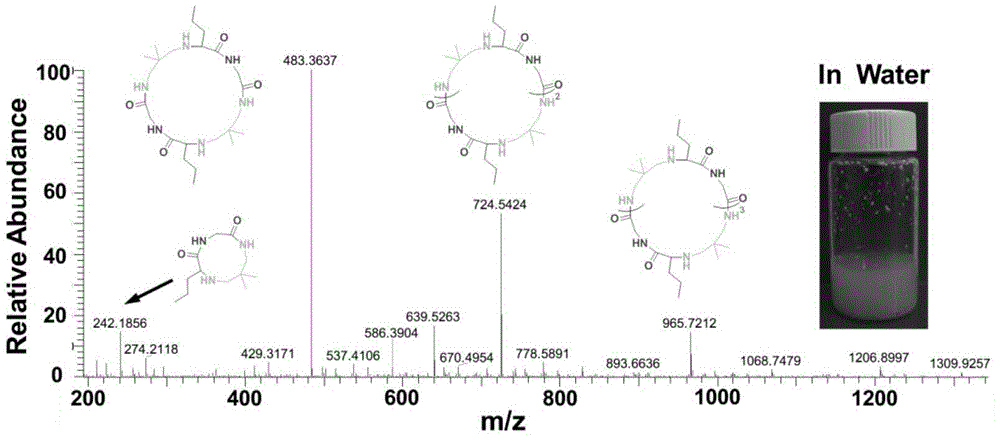
[0038] Aqueous phase: Add 5 ml of water to a 20 ml vial, and argon for ten minutes to remove oxygen in the water. Then add 20% of the total mass of the reactants (diamine, isonitrile, aldehyde) to the solution and add 20% of the total mass of the Candida antarctic lipase, 1 millimole of n-butyraldehyde and 1 millimole of 2,2- Dimethyl-1,3-propanediamine was stirred at room temperature for 15 minutes. After the reaction solution was completely clear, 1 mmol of ethyl isonitrile was added to the reaction solution, and the reaction solution was stirred at room temperature for 48 hours.
[0039] The reaction solution was characterized by mass spectrometry, such as figure 2 As shown, the four regular signal peaks 242.2, 483.4, 724.5 and 965.7 correspond to cyclic peptides of corresponding molecular weights, and there are very few impurity ion peaks.
[0040] The structure and yield of the formed cyclic peptide are shown in Table 1. With mass spectrometry, the product in water is almost...
Embodiment 3
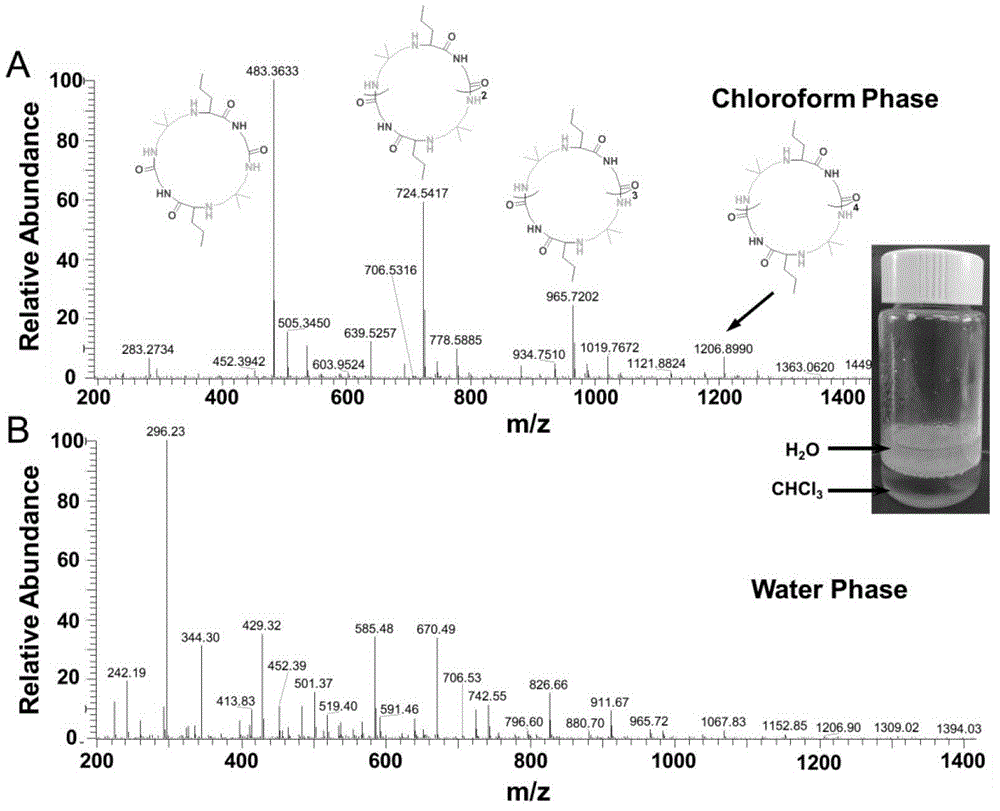
[0042] Chloroform / water mixed phase: Add 5 ml of chloroform / water (volume ratio 1:1) into a 20 ml vial, and let argon gas for ten minutes to remove oxygen in the solution. Then add 20% of the total mass of the reactants (diamine, isonitrile, aldehyde) of Candida antarctica lipase, 1 mmol of n-butyraldehyde and 1 mmol of 2,2-dimethyl-1 to the solution , 3-propanediamine, and stir at room temperature for 15 minutes. After the reaction solution was completely clear, 1 mmol of ethyl isonitrile was added to the reaction solution, and the reaction solution was stirred at room temperature for 48 hours. After the reaction, the water layer of the reaction solution was a bright yellow clear solution, and the chloroform layer was a light yellow clear solution. Separate the reaction solution and lipase, and directly analyze the composition of the aqueous phase and the chloroform phase by mass spectrometry. No HPLC purification.
[0043] The reaction solution was characterized by mass spec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com