Preparation method of ethoxyquin serving as antioxidant
A technology of ethoxyquinoline and antioxidant, applied in the chemical industry, can solve problems affecting product quality, high reaction cost, low conversion rate, etc., and achieve the effects of reducing human damage, reducing environmental pollution, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
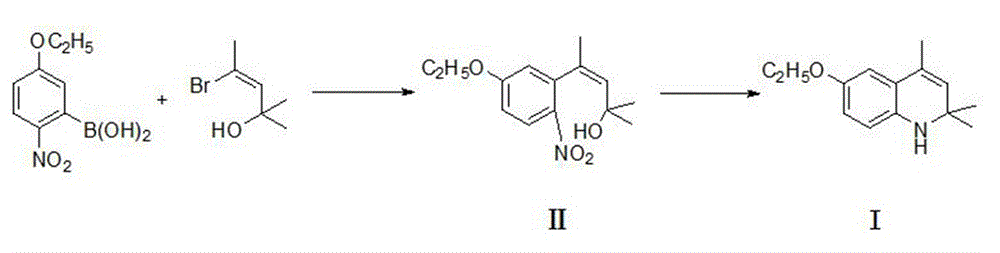
[0016] A. Add 211g of 2-nitro-5-ethoxyphenylboronic acid, 2110gDMF, 118g sodium carbonate and 5g catalyst tetrakistriphenylphosphine palladium in the reactor, stir well and then add dropwise 196.9g4-bromo-2- Methyl-2-hydroxy-3-pentene; after the addition, the temperature was raised to 70°C for 12 hours. After the reaction, the reaction solution was naturally cooled to room temperature; the reaction solution was filtered to remove insoluble matter, and the filtrate was distilled under reduced pressure to remove solvent, and then pour the distillation residue into an ice-water mixture, and collect the precipitated solid by filtration, which is the crude product 4-(5-ethoxy-2-nitrophenyl)-2-methyl-2-hydroxy- 3-Pentene, the crude product was recrystallized by ethanol to obtain 199.2g light yellow solid, which was the refined product 4-(5-ethoxy-2-nitrophenyl)-2-methyl-2-hydroxy- 3-pentene, the yield is about 75.2% (calculated using 2-nitro-5-ethoxyphenylboronic acid as raw materia...
Embodiment 2
[0020] A. add the 2-nitro-5-ethoxyphenylboronic acid of 211g in the reactor, the DMF of 1266g, 106g sodium carbonate and 5g catalyst tetraphenylphosphine palladium, stir the 4-bromo of 177g again dropwise -2-Methyl-2-hydroxy-3-pentene, after the addition is completed, the temperature is raised to 70 ° C for 8 hours, after the reaction, the reaction solution is naturally cooled to room temperature; the reaction solution is filtered to remove insoluble matter, and the filtrate is subjected to vacuum distillation After most of the solvent was evaporated, the distilled product was poured into ice water, and the precipitated solid was collected by filtration as the crude product 4-(5-ethoxy-2-nitrophenyl)-2-methyl-2-hydroxy-3 -pentene, the crude product was recrystallized from ethanol to obtain 172.5g of a light yellow solid, which is the refined product 4-(5-ethoxy-2-nitrophenyl)-2-methyl-2-hydroxy-3-pentene , the yield is about 65.1%;
[0021] B. Step B of this embodiment is the...
Embodiment 3
[0023] A. Add 211g of 2-nitro-5-ethoxyphenylboronic acid, 1500g of DMF, 108g of sodium carbonate and 5g of catalyst tetraphenylphosphine palladium into the reactor, stir well and then add 4-bromo-2-methyl -2-Hydroxy-3-pentene 198g, heat up to 70°C for 10 hours after the addition, after the reaction, let the reaction solution cool down to room temperature naturally, filter the reaction solution to remove insoluble matter, take the filtrate and distill most of it under reduced pressure After the solvent was removed, the residual distillation product was poured into ice water, and the precipitated solid crude product 4-(5-ethoxy-2-nitrophenyl)-2-methyl-2-hydroxy-3-pentene was collected by filtration, Crude 4-(5-ethoxy-2-nitrophenyl)-2-methyl-2-hydroxy-3-pentene was passed through ethanol Heavy The 192.6g light yellow solid obtained after crystallization is the refined product 4-(5-ethoxy-2-nitrophenyl)-2-methyl-2-hydroxy-3-pentene, and the yield is about 72.7% ;
[0024] B. St...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com