Method for producing ethoxyquin
An ethoxyquinoline and production method technology, applied in directions such as organic chemistry, can solve the problems of long reaction time, many side reactions, long production cycle and the like, and achieve the effects of shortening production cycle, shortening reaction time, and shortening reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
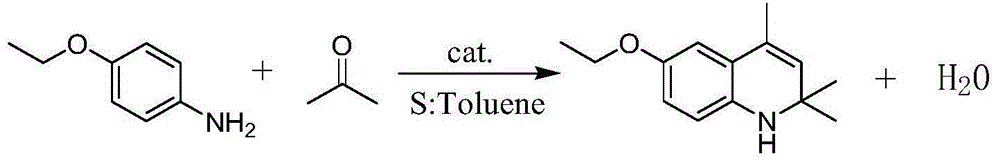
[0028] Add 20g of p-aminophenetole, 5.5mL of toluene, 1.1g of p-toluenesulfonic acid and 0.42g of iodine into the reactor equipped with a thermometer, a water separator and a condenser. Heat and stir with a heat-collecting magnetic stirrer. After the temperature of the reaction solution reaches 130° C., start to add 30 mL of acetone dropwise into the reactor through a syringe pump, and the dropwise addition is completed within 9 hours. After the dropwise addition of acetone was completed, the reaction was terminated. Add 3% aqueous sodium bicarbonate solution to the reaction solution for neutralization, then pour into the separatory funnel and let stand for layering. After the organic phase is washed with water, it is distilled under reduced pressure to obtain the target product ethoxyquinoline with a yield of 85.9%. %.
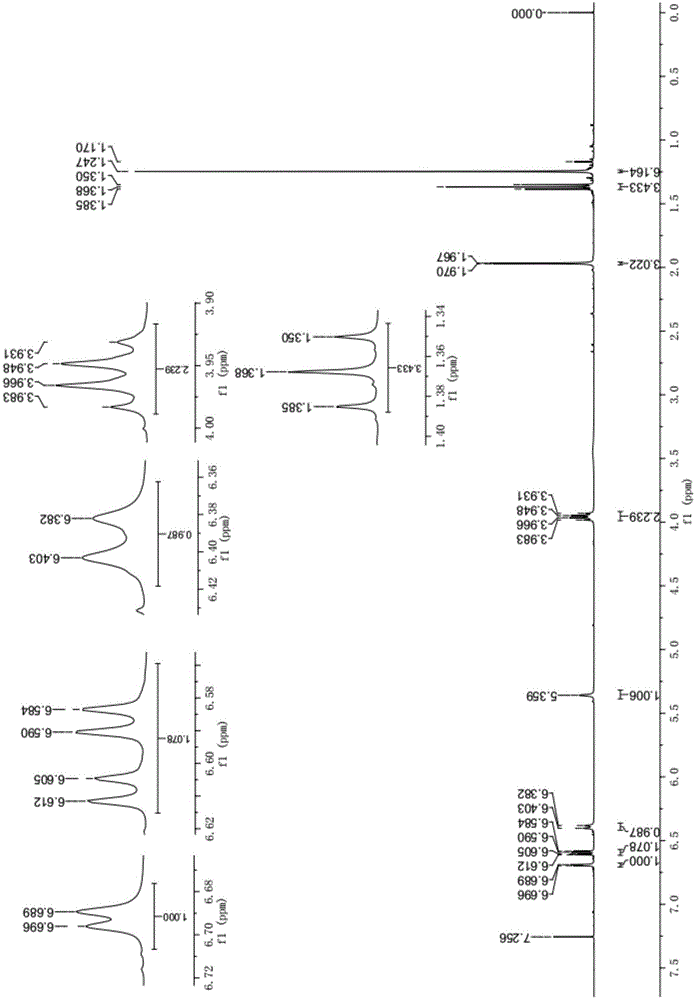
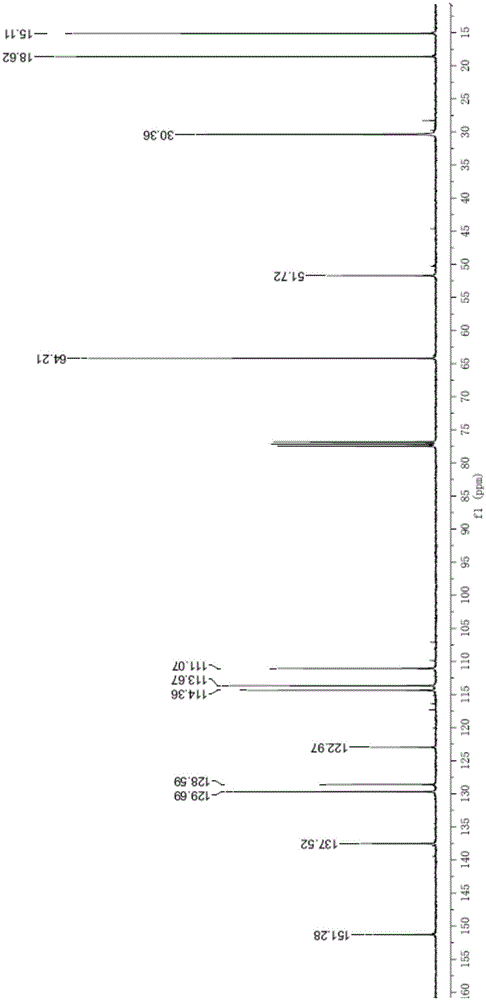
[0029] see figure 1 and 2 , the results of the proton nuclear magnetic resonance spectrum and the carbon nuclear magnetic resonance spectrum of the presen...
Embodiment 2
[0033] Add 20g of p-aminophenetole, 6.5mL of toluene, 1.3g of p-toluenesulfonic acid and 0.55g of iodine into a reactor equipped with a thermometer, a water separator and a condenser. Heat and stir with a heat-collecting magnetic stirrer. After the temperature of the reaction solution stabilizes to 130° C., start to add 32 mL of acetone dropwise into the reactor through a syringe pump, and the dropwise addition is completed within 10 hours. After the dropwise addition of acetone was completed, the reaction was terminated. Add 3% aqueous sodium bicarbonate solution to the reaction solution for neutralization, then pour into the separatory funnel and let stand for layering. After the organic phase is washed with water, it is distilled under reduced pressure to obtain the target product ethoxyquinoline with a yield of 91.6%. %.
Embodiment 3
[0035] Add 20g of p-aminophenetole, 8mL of toluene, 1.8g of p-toluenesulfonic acid and 0.27g of iodine into the reactor equipped with thermometer, water separator and condenser. Heat and stir with a heat-collecting magnetic stirrer. After the temperature of the reaction solution stabilizes to 130° C., start to add 32 mL of acetone dropwise into the reactor through a syringe pump, and the dropwise addition is completed within 10 hours. After the dropwise addition of acetone was completed, the reaction was terminated. Add 3% sodium bicarbonate aqueous solution in the reaction solution and carry out neutralization, then pour in the separating funnel and stand layering, carry out vacuum distillation after the organic phase washes with water, obtain target product ethoxyquinoline, productive rate is 88.7% %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com