Budesonide preparing method
A technology for re-refining and compounding, applied in the field of preparation of budesonide raw materials, can solve the problems of cumbersome operation, unfavorable industrial production, severe reaction conditions, etc., and achieve the effects of mild reaction conditions, great application value, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
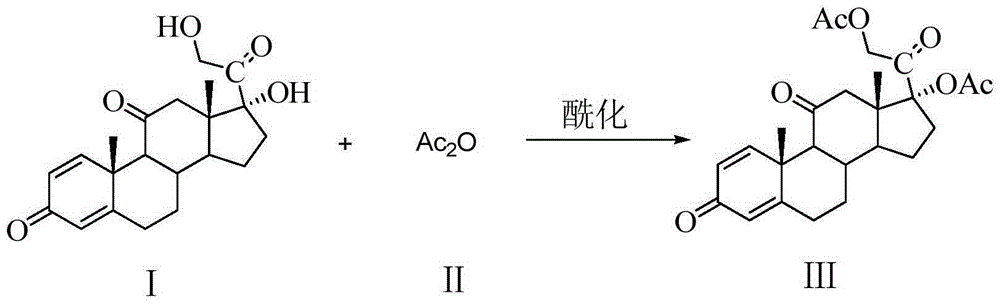
[0049] Example 1: Preparation of 17,21-diacetoxy-1,4-pregnadiene-3,11,20-trione III
[0050]
[0051] Add 200 g (0.56 mol) of prednisone and 1 L of pyridine into a 3 L three-necked round-bottomed flask, stir to dissolve, add 150 mL of acetic anhydride, react at 85°C to 90°C for 2.5 hours, monitor the reaction by TLC until the reaction is complete. The reactant was poured into 10 L of ice water, left to solidify for 2.5 h, filtered with suction, washed with water, and dried to constant weight to obtain 243 g of intermediate compound III as a white solid, which was directly used in the next step. The yield was 98.4%, and the chemical purity by HPLC was 98.2%.
Embodiment 2
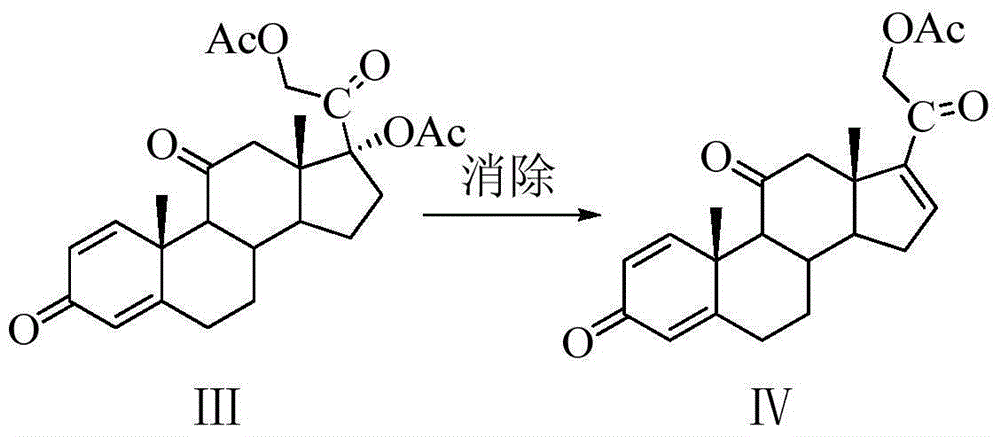
[0052] Example 2: Preparation of 21-acetoxy-1,4,16-pregnatriene-3,11,20-trione IV
[0053]
[0054] Add 240g (0.54mol) of 17,21-diacetoxy-1,4-pregnadiene-3,11,20-trione III, 120g of anhydrous sodium acetate and 1.5L of DMF into a 3L three-neck round bottom flask , under the protection of nitrogen, react at 95° C. to 100° C. for 4.5 h, and monitor the reaction by TLC until the reaction is complete. The reactant was poured into 15 L of ice water, allowed to stand for 3 hours to solidify, filtered with suction, washed with water, and dried to a constant weight to obtain 200 g of intermediate compound IV as a khaki solid, which was directly used in the next step. The yield was 96.4%, and the chemical purity by HPLC was 97.8%.
Embodiment 3
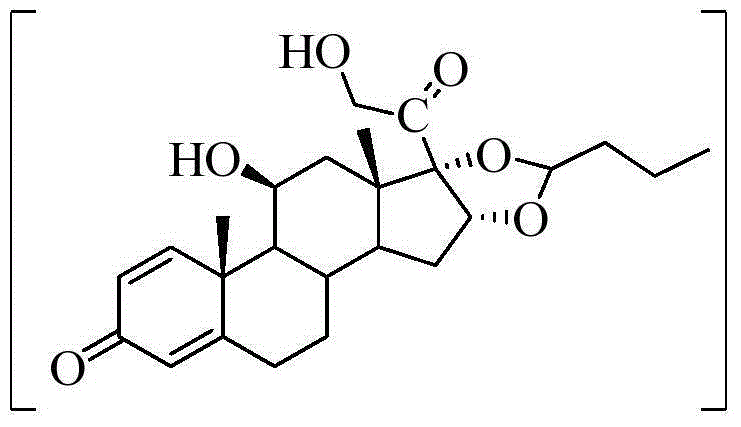
[0055] Example 3: Preparation of 16α, 17α-dihydroxy-21-acetoxy-1,4-pregnadiene-3,11,20-trione V
[0056]
[0057] Add 200g (0.52mol) of 21-acetoxy-1,4,16-pregnatriene-3,11,20-trione IV and 6L of acetone into a 10L stainless steel reaction tank, stir to dissolve, and control in an ice-water bath. The inner temperature is 0°C-5°C; 115g of potassium permanganate is firstly dissolved in 800mL of water, and in an ice-water bath, when the inner temperature is 0°C-5°C, add 800mL of frozen acetone and 50mL of frozen formic acid, and stir evenly; The potassium solution was added to the acetone solution of the intermediate compound IV, and the reaction was rapidly stirred at 0°C to 5°C for 7 minutes, and the reaction was quenched with 600 mL of 15% aqueous sodium sulfite solution, filtered with suction, the filter cake was washed with acetone, and the mother liquor was concentrated and evaporated to remove the acetone. A solid precipitated, filtered, washed the filter cake with water...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com