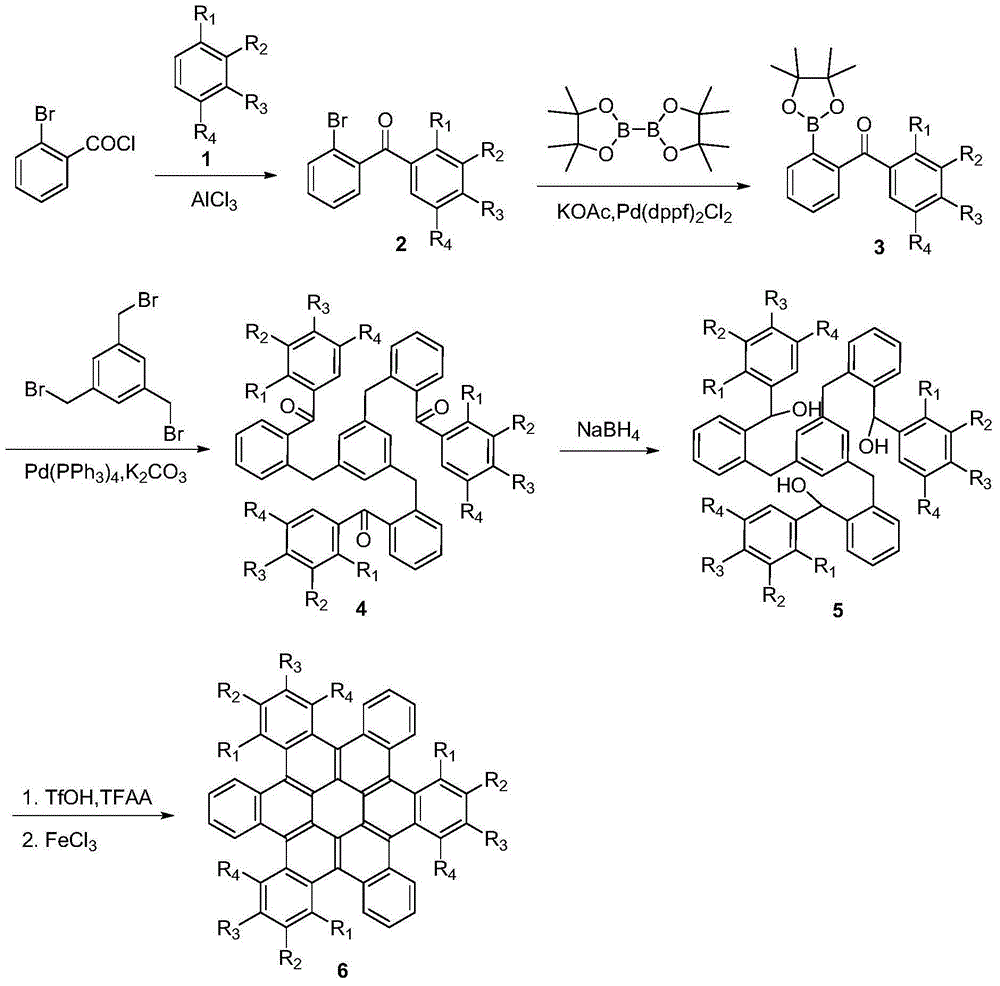
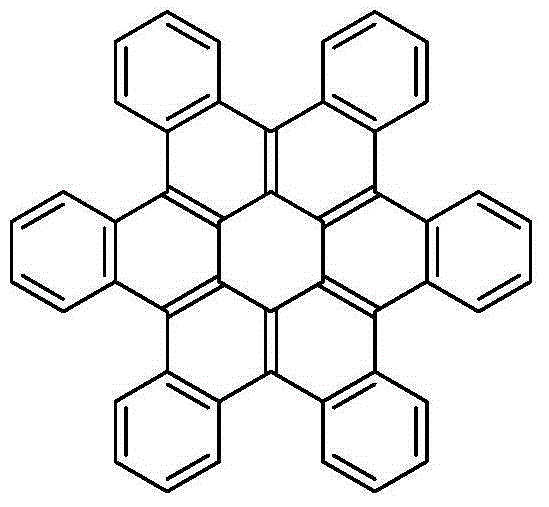
Method for synthesis of hexa-cata-hexabenzocoronene and derivatives thereof
A technology of benzophenone and its derivatives, which is applied in the field of synthesis of hexabenzophenone compounds and their derivatives, achieving the effects of mild reaction conditions and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Taking the synthesis of hexabenzophenone with the following structural formula as an example, the specific synthesis method is as follows:
[0028]
[0029] 1. Friedel-Crafts acylation reaction
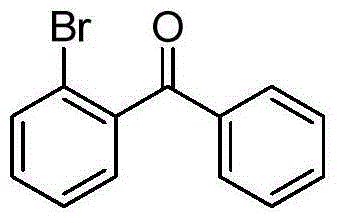
[0030] Add 2.19g (10mmol) of o-bromobenzoyl chloride and 50mL of dry dichloromethane to a 100mL three-necked flask, and add 1.33g (10mmol) of aluminum trichloride and 8.59g (11mmol) of benzene at 0°C under the protection of argon. , stirred at room temperature for 3 hours, quenched the reaction with saturated ammonium chloride, extracted three times with dichloromethane, combined the organic phases, dried with anhydrous sodium sulfate, and removed the solvent by rotary evaporation. The volume ratio of petroleum ether to ethyl acetate was The 10:1 mixed solution was used as a developing solvent to separate the product by column chromatography to obtain 2-bromobenzophenone with the following structural formula, and the yield was 98.2%.
[0031]
[0032] 2. Boronation react...
Embodiment 2
[0048] Taking the synthesis of 2,10,18-trimethylhexabenzophenone with the following structural formula as an example, the specific synthesis method is as follows:
[0049]
[0050] 1. Friedel-Crafts acylation reaction
[0051] Add 2.19g (10mmol) of o-bromobenzoyl chloride and 50mL of dry dichloromethane into a 100mL three-necked flask, add 1.33g (10mmol) of anhydrous aluminum trichloride at 0°C under the protection of argon, and stir the reaction until the trichloride Aluminum chloride was completely dissolved, then added 1.01g (11mmol) toluene to the mixed solution, stirred and reacted at 0°C for 3 hours, the solution turned from yellow to orange, TLC tracked and monitored no raw material point, quenched the reaction with saturated aqueous ammonium chloride solution to Neutral, extracted three times with dichloromethane, combined the organic phases, dried over anhydrous sodium sulfate, removed the solvent by rotary evaporation, and used the mixed solution of petroleum ethe...
Embodiment 3
[0069] Taking the synthesis of 2,10,18-trimethoxyhexabenzophenone with the following structural formula as an example, the specific synthesis method is as follows:
[0070]
[0071] 1. Friedel-Crafts acylation reaction
[0072] In step 1 of Example 2, the toluene used was replaced with equimolar anisole, and the reaction was stirred at 0°C for 2 hours. The other steps were the same as in Example 2 to obtain a white powder compound (2-bromophenyl ) (4-methoxyphenyl)methanone with a yield of 90.5%.
[0073]
[0074] 2. Boronation reaction
[0075] In step 2 of Example 1, the 2-bromobenzophenone used is replaced with equimolar (2-bromophenyl) (4-methoxyphenyl) ketone, and other steps are the same as in Example 1, A white powdery solid compound 2-(4-methoxy)benzoylphenylboronic acid pinacol ester with the following structural formula was obtained with a yield of 90.7%.
[0076]
[0077] 3. Coupling reaction
[0078] In step 3 of Example 1, the 2-benzoylphenylboronic a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com