Preparation of lisinopril sustained-release tablets
A technology of sustained-release tablets and weight percentage, which is applied in the field of preparation of lisinopril sustained-release tablets, can solve the problems of poor reproducibility, complex process, unstable release rate and the like, and achieves the effects of uniform drug release rate and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
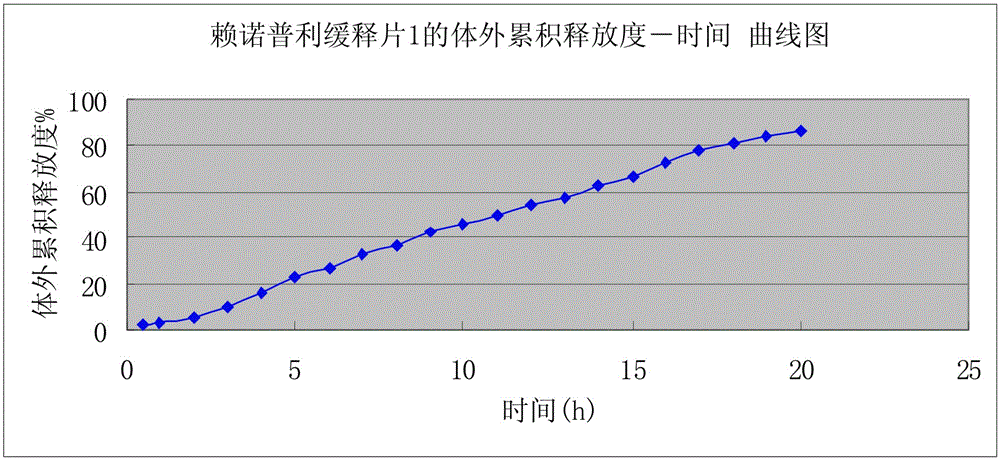
[0021] A kind of preparation of lisinopril sustained-release tablet, the weight of described lisinopril sustained-release tablet is 1g In the present embodiment, the following consumption containing lisinopril sustained-release tablet raw material is calculated according to the amount of 1000, described The raw materials and weight percent of lisinopril sustained-release tablets are: lisinopril 10g, skeleton material 660g, pregelatinized starch 180g, microcrystalline cellulose 142g, magnesium stearate 8g; Mu; the preparation of the lisinopril sustained-release tablet, according to the above weight percentage, lisinopril is pulverized and added in a three-dimensional motion mixer after passing through a 100 mesh sieve, carbomer, pregelatinized starch, microcrystalline cellulose , magnesium stearate pulverize and cross 80 mesh sieves and add three-dimensional kinematic mixer; Lisinopril sustained-release tablets; when pressing tablets, the hardness of the tablet needs to be cont...
Embodiment 2
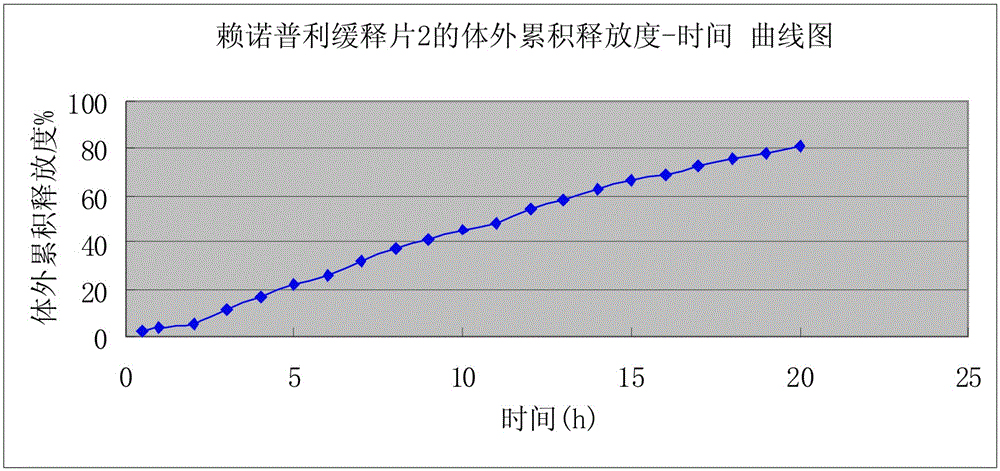
[0023] A kind of preparation of lisinopril sustained-release tablet, the weight of described lisinopril sustained-release tablet is 1g In the present embodiment, the following consumption containing lisinopril sustained-release tablet raw material is calculated according to the amount of 1000, described The raw materials and weight percent of lisinopril sustained-release tablets are: 20g of lisinopril, 600g of skeleton material, 220g of pregelatinized starch, 154g of microcrystalline cellulose, 6g of magnesium stearate; Mu; the preparation of the lisinopril sustained-release tablet, according to the above weight percentage, lisinopril is pulverized and added in a three-dimensional motion mixer after passing through a 100 mesh sieve, carbomer, pregelatinized starch, microcrystalline cellulose , magnesium stearate pulverize and cross 80 mesh sieves and add three-dimensional kinematic mixer; Lisinopril sustained-release tablets; when pressing tablets, the hardness of the tablet n...
Embodiment 3
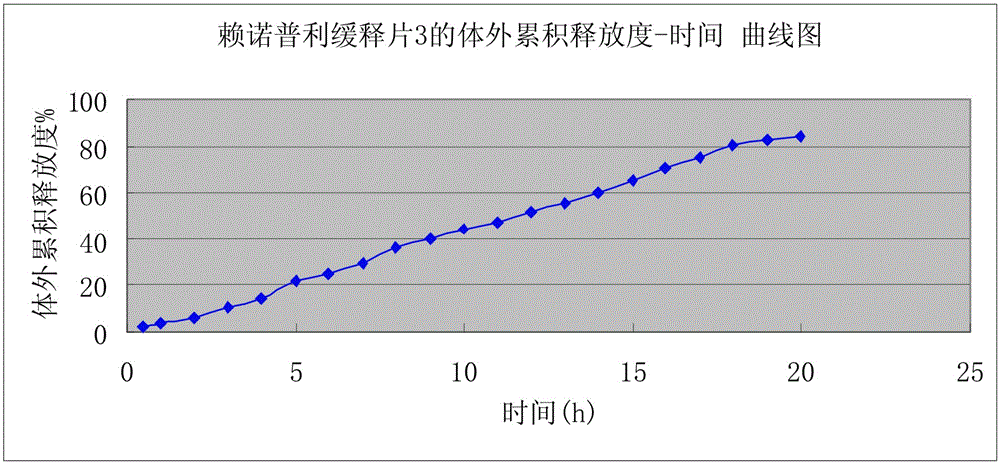
[0025] A kind of preparation of lisinopril sustained-release tablet, the weight of described lisinopril sustained-release tablet is 1g In the present embodiment, the following consumption containing lisinopril sustained-release tablet raw material is calculated according to the amount of 1000, described The raw materials and weight percentages of lisinopril sustained-release tablets are: 30g of lisinopril, 570g of skeleton material, 230g of pregelatinized starch, 165g of microcrystalline cellulose, and 5g of magnesium stearate; Mu; the preparation of the lisinopril sustained-release tablet, according to the above weight percentage, lisinopril is pulverized and added in a three-dimensional motion mixer after passing through a 100 mesh sieve, carbomer, pregelatinized starch, microcrystalline cellulose , magnesium stearate pulverize and cross 80 mesh sieves and add three-dimensional kinematic mixer; Lisinopril sustained-release tablets; when pressing tablets, the hardness of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com