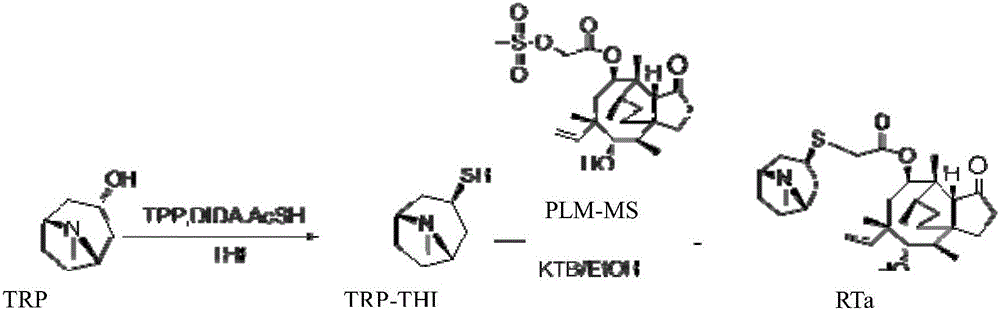
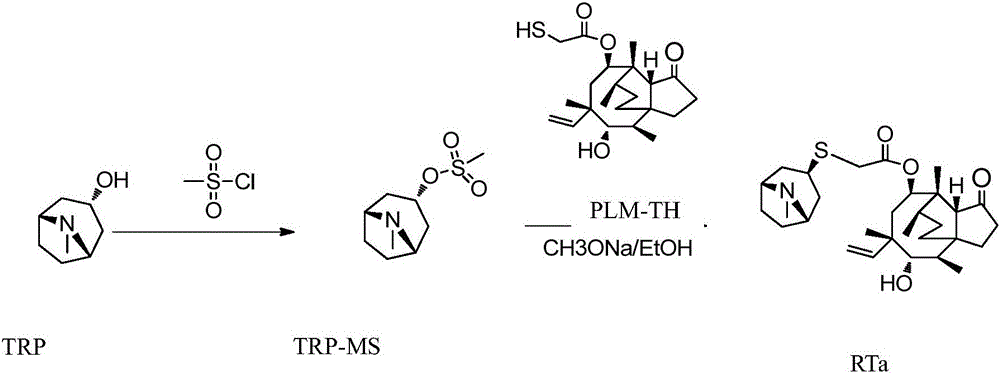
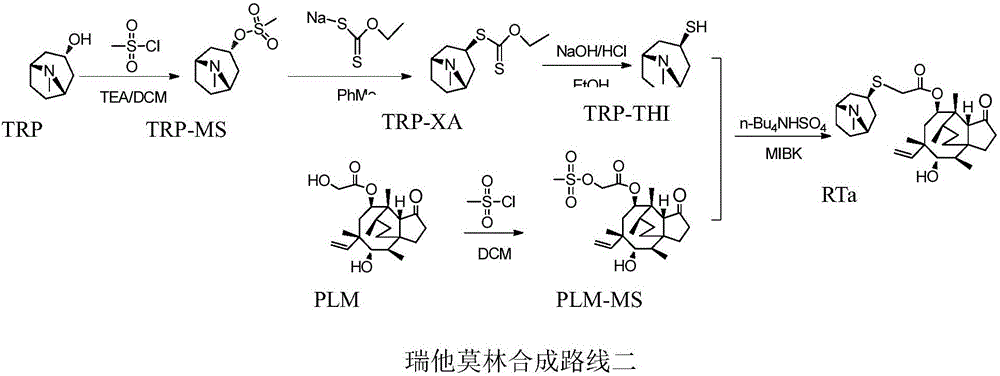
A kind of retapamulin synthetic method
A technology of retamoline and a synthesis method, applied in the field of pharmaceutical antibiotics, can solve the problems of inability to guarantee the quality of the final product, increase the difficulty of processing, and difficult to control the purity of the intermediates of retamoline in each step, and achieve significant economic value. , The effect of low production cost and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0054] Step a: Preparation of Pleuromutilin Mesylate (PLM-TS)
[0055] Weigh 189.25g (0.5mol) of pleuromutilin, 104.86g (0.55mol) of p-toluenesulfonyl chloride was dissolved in 946mL of dichloromethane, and 55.65g of p-triethylamine (dissolved in 55mL of dichloromethane) was added dropwise at 10°C. ), after the dropwise addition was completed, the reaction was continued at 10°C for 2 hours, the reaction solution was washed once with 500mL of water and once with 250mL of saturated brine, spin-dried in dichloromethane, and recrystallized with absolute ethanol to obtain 253.03g of PLM-TS. Yield 95.0%.
[0056] Step b: Preparation of Tropinol Mesylate (TRP-MS)
[0057] Weigh 200.0g (1.42mol) of tropinol, 158.1g (1.56mol) of triethylamine and dissolve in 1000mL of dichloromethane solution, cool to -10~0°C, add dropwise 178.9g of methanesulfonyl chloride ( Dissolved in 200mL dichloromethane), after the dropwise addition, keep this temperature to continue the reaction for 2 hours, ...
example 2
[0065] Step a: Preparation of Pleuromutilin Mesylate (PLM-TS)
[0066] Weigh 325.0g (0.86mol) of pleuromutilin, 213.15g (1.12mol) of p-toluenesulfonyl chloride was dissolved in 1625mL of dichloromethane, and 113.13g of p-triethylamine (dissolved in 100mL of dichloromethane) was added dropwise at 40°C. ), after the dropwise addition was completed, the reaction was continued at 40°C for 6 hours, the reaction solution was washed once with 800mL water, once with 400mL saturated brine, spin-dried in dichloromethane, and recrystallized with absolute ethanol to obtain 445.3g of PLM-TS, Yield 97.2%.
[0067] Step b: Preparation of Tropinol Mesylate (TRP-MS)
[0068] Weigh 400.0g (2.84mol) of tropinol, and dissolve 477.2g (3.69mol) of diisopropylethylamine in 2000mL of dichloromethane solution, cool down to -10~0°C, add methanesulfonyl chloride dropwise under nitrogen protection 422.9g (dissolved in 400mL dichloromethane), after the dropwise addition, keep this temperature to continu...
example 3
[0076] Step a: Preparation of Pleuromutilin Mesylate (PLM-TS)
[0077] Weigh 378.5.0g (1.0mol) of pleuromutilin, 228.78g (1.2mol) of p-toluenesulfonyl chloride was dissolved in 1892mL of dichloromethane, and 121.43g of p-triethylamine (dissolved in 120mL of dichloromethane middle), after the dropwise addition was completed, the reaction was continued at 30°C for 4 hours, the reaction solution was washed once with 1000mL water, once with 500mL saturated brine, spin-dried in dichloromethane, and recrystallized with absolute ethanol to obtain 523.6g of PLM-TS , yield 98.3%.
[0078] Step b: Preparation of Tropinol Mesylate (TRP-MS)
[0079] Weigh 500.0g (3.54mol) of tropinol, and dissolve 549.1g (4.25mol) of diisopropylethylamine in 2500mL of dichloromethane solution, cool down to -10-0°C, and add methanesulfonyl chloride dropwise under nitrogen protection 486.8g (dissolved in 500mL dichloromethane), after the dropwise addition, keep this temperature to continue the reaction for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com