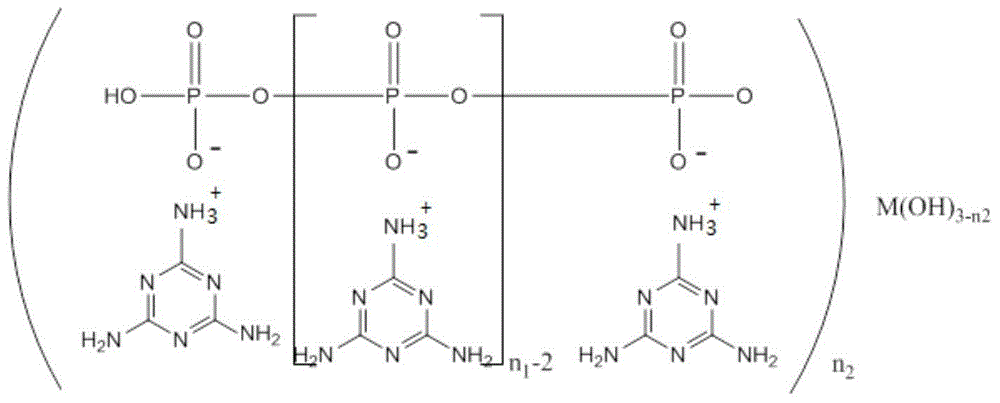
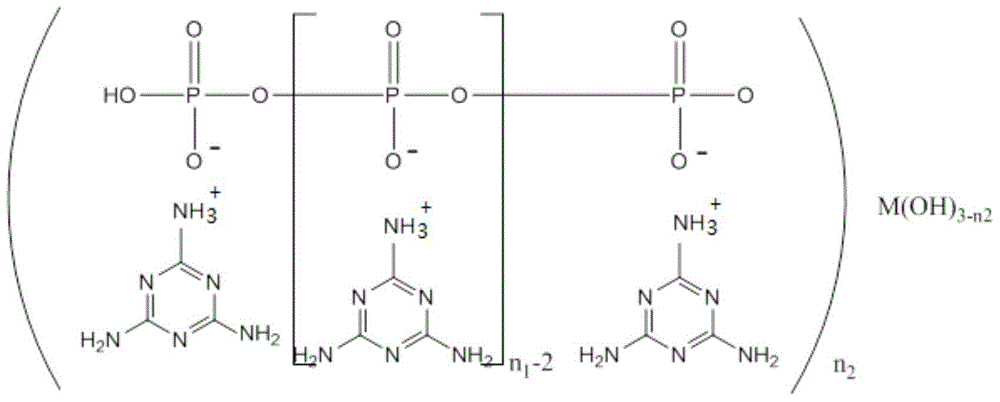
Melamine polyphosphoric acid rare earth metal salt, synthetic method and application
A technology of rare earth and rare earth metal salts of melamine polyphosphate, which is applied in the direction of organic chemistry, can solve the problems of environmental protection and hygienic restrictions, and achieve the effects of improving thermal stability, high flame retardant efficiency, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
[0041] Embodiment 1 Melamine polyphosphate lanthanum salt
[0042] 12.5g of melamine and 9.9g of lanthanum oxide were added to a 500ml four-neck flask filled with 300ml of distilled water, a stirring bar and a thermometer, and the temperature was slowly raised to 80°C under stirring, and 4.6g of hydrochloric acid (37%wt) was added dropwise to the flask, measured The pH value of the solution was about 6.0. After stirring for 1.0 h, 22.2 g of sodium pyrophosphate was added. After reacting for 1 h at 80° C., 16.5 g of hydrochloric acid (37% wt) was added dropwise to the flask for the second time, and the pH value of the solution was measured to be about 6.0. 2.0, continue to react for 1.0 h, cool to room temperature, filter, centrifuge, wash with distilled water until neutral, and dry to obtain lanthanum melamine polyphosphate with a yield of 83%. After elemental analysis, the content of lanthanum element was 13.5%.
Embodiment 2 3
[0043] Embodiment 2 Melamine polyphosphate cerium salt
[0044] 12.5g of melamine and 9.7g of cerium chloride were added to a 500ml four-neck flask filled with 300ml of distilled water, a stirring bar and a thermometer, and the temperature was slowly raised to 90°C under stirring, and 4.8g of hydrochloric acid (37%wt) was added dropwise to the flask. The pH value of the solution was measured to be about 5.0. After stirring for 0.5 h, 22.2 g of sodium pyrophosphate was added. After reacting for 0.5 h at 90° C., 16.5 g of hydrochloric acid (37% wt) was added dropwise to the flask for the second time, and the pH of the solution was measured. The value is about 2.0, continue to react for 1.5h, cool to room temperature, filter, centrifuge, wash with distilled water until neutral, and dry to obtain cerium melamine polyphosphate with a yield of 85%. According to elemental analysis, the content of cerium element is 13.4%.
Embodiment 3 3
[0045] Embodiment 3 melamine polyphosphate praseodymium salt
[0046] 12.5g of melamine and 5.9g of praseodymium hydroxide were added to a 500ml four-neck flask filled with 300ml of distilled water, a stirring bar and a thermometer, and the temperature was slowly raised to 90°C under stirring, and 4.6g of hydrochloric acid (37%wt) was added dropwise to the flask. The pH value of the solution was measured to be about 6.0. After stirring for 0.5 h, 22.2 g of sodium pyrophosphate was added. After reacting for 0.5 h at 90° C., 16.3 g of hydrochloric acid (37% wt) was added dropwise to the flask for the second time, and the pH of the solution was measured. The value was about 3.0, continued to react for 1.5h, cooled to room temperature, filtered, centrifuged, washed with distilled water until neutral, and dried to obtain praseodymium melamine polyphosphate with a yield of 86%. According to elemental analysis, the content of praseodymium element is 13.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com