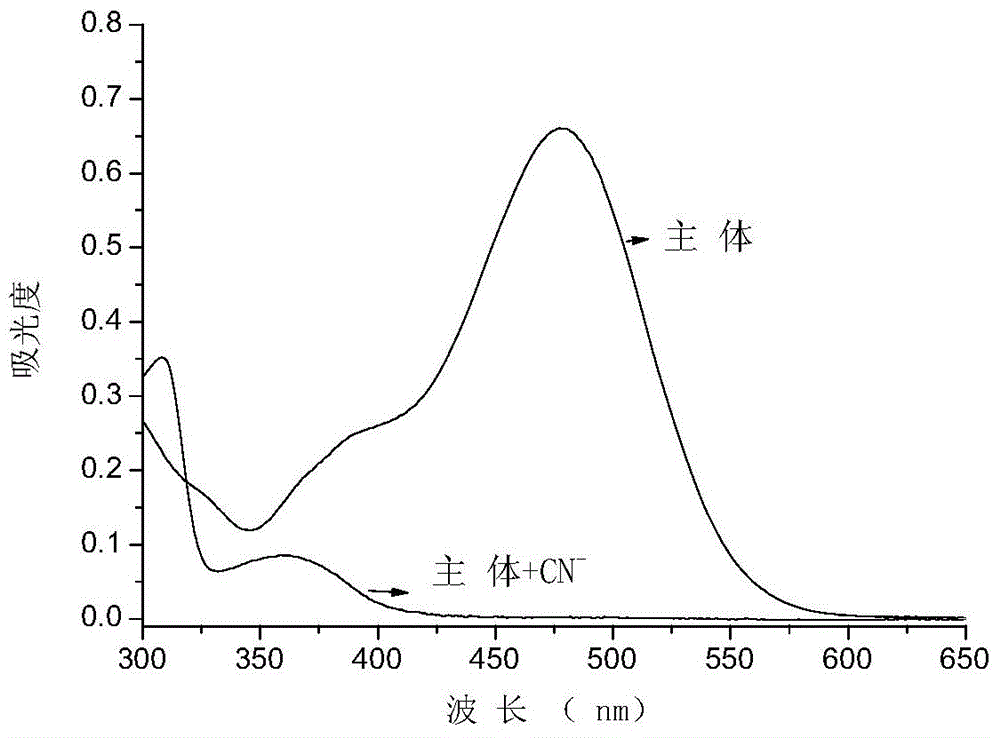
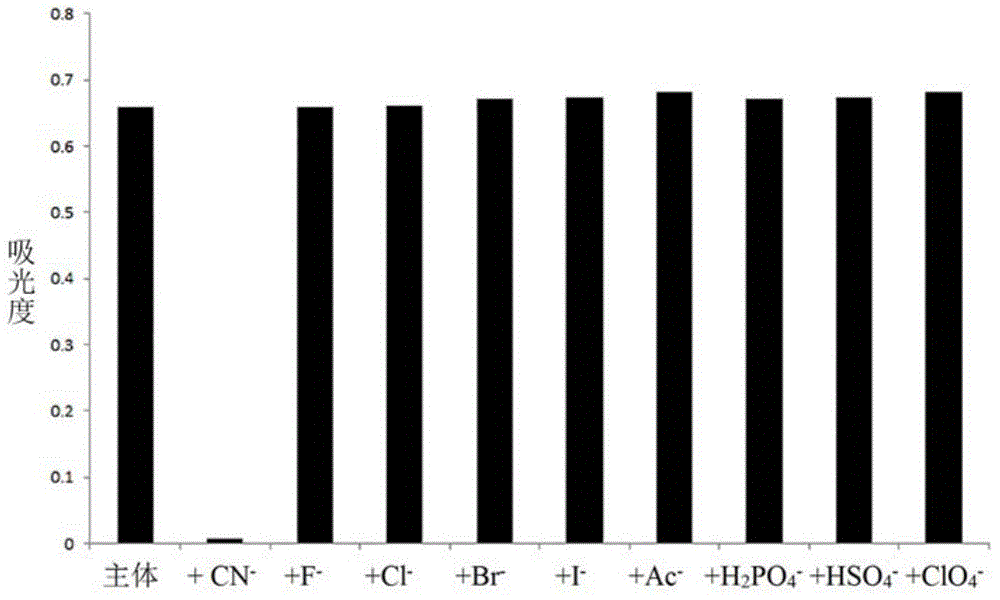
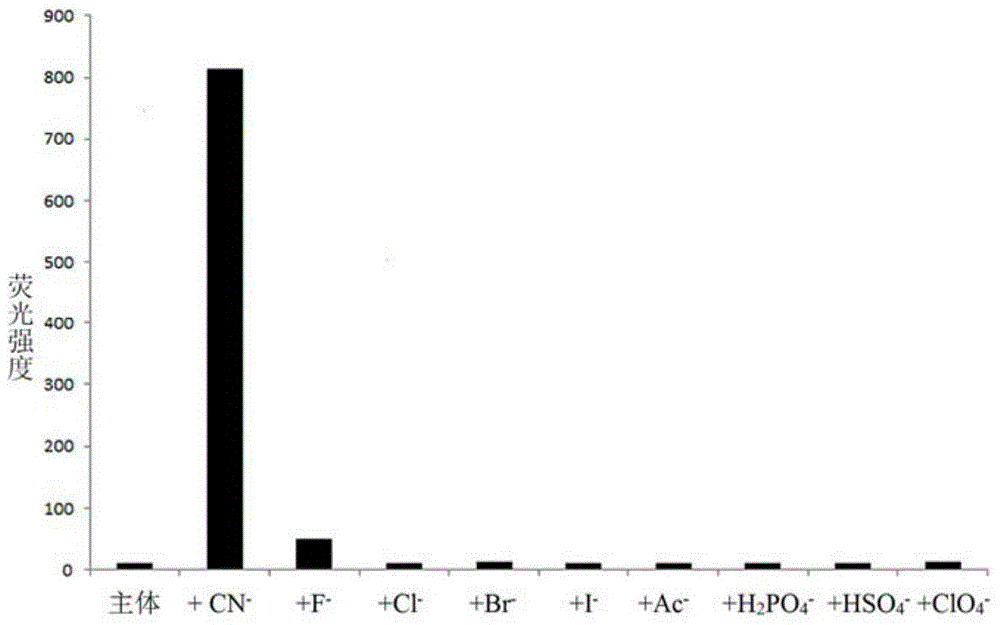
Cyanide receptor compound based on 2-cyano-3-(6-N, N-dimethylamino-2-naphthyl) acrylonitrile, preparation method and application
A technology of dimethylammonia and compounds, which is applied in the field of anion detection, can solve the problems of complex receptor structure, difficulty in synthesis, and restrictions on the application of detection and identification of cyanide ions, and achieve the effect of easy-to-obtain raw materials and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] (2) CN - Preparation of ion detection test paper
[0034] For convenient and quick detection of CN - , the present invention has prepared the cyanide detection test paper based on this acceptor. The specific preparation method is as follows:
[0035] First filter paper with 0.1 ~ 0.5mol L -1 Soak in dilute hydrochloric acid for 0.5 to 1 hour, wash with distilled water until the filtrate is neutral; remove the water by suction filtration, and dry the filter paper in a vacuum oven; then dissolve the acceptor compound in HEPES-DMSO- h 2 In O system, the concentration is 1.0~2.0×10 -3 mol L -1 The solution was added dropwise to the treated filter paper to make the HEPES-DMSO-H of the acceptor compound 2 The O solution system is evenly adsorbed on the filter paper; then the filter paper is dried in a vacuum oven, and finally cut into 2cm×2cm test paper pieces to obtain CN - Ion detection test paper.
[0036] Cyanide detection test paper detection CN - The method is a...
Embodiment 1
[0041] 1. Receptor synthesis
[0042] Mix 6-dimethylamino-2-naphthaldehyde (2mmol) and malononitrile (2.2mmol) in absolute ethanol (20mL), add piperidine (0.04mmol), stir at room temperature for 3h, cool down after the reaction After suction filtration, a red solid was obtained, which was washed several times with absolute ethanol to obtain the product—2-cyano-3-(6-N,N-dimethylamino-2-naphthyl)acrylonitrile.
[0043] Yield: 82.0% (m.p.170℃~172℃), IR: (KBr,cm -1 )v: 2213(-CN), 1619(C=C). 1 HNMR (500MHz, DMSO) δ8.37(s, 1H), 8.24(s, 1H), 7.95(d, J=8.8Hz, 1H), 7.85(d, J=9.2Hz, 1H), 7.73(dd, J=12.5,6.2Hz,1H),7.30(dd,J=9.2,2.4Hz,1H),6.98(d,J=1.9Hz,1H),3.12(s,6H). 13 CNMR (DMSO-d 6 ,125MHz):δ160.9,151.7,138.4,136.3,131.7,127.5,127.3,124.8,124.6,117.3,115.9,115.0,105.4,75.9,40.88,40.82; MSm / z[M] + Calcd for C 16 h 13 N 3 247.1, found 247.4.
[0044] 2. CN - Preparation and detection of test paper
[0045] (1) CN - Preparation of test paper: cut the filter paper into a squa...
Embodiment 2
[0048] 1. Mix 6-dimethylamino-2-naphthaldehyde (2mmol) and malononitrile (2mmol) in absolute ethanol (20mL), add piperidine (0.04mmol), stir at room temperature for 3h, after the reaction After cooling and suction filtration, a red solid was obtained, which was rinsed several times with absolute ethanol to obtain the product—2-cyano-3-(6-N,N-dimethylamino-2-naphthyl)acrylonitrile.
[0049] Yield: 80%. The characterization data of the synthesized product are the same as in Example 1.
[0050] 2. CN - Preparation and detection of detection test paper: same as embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com