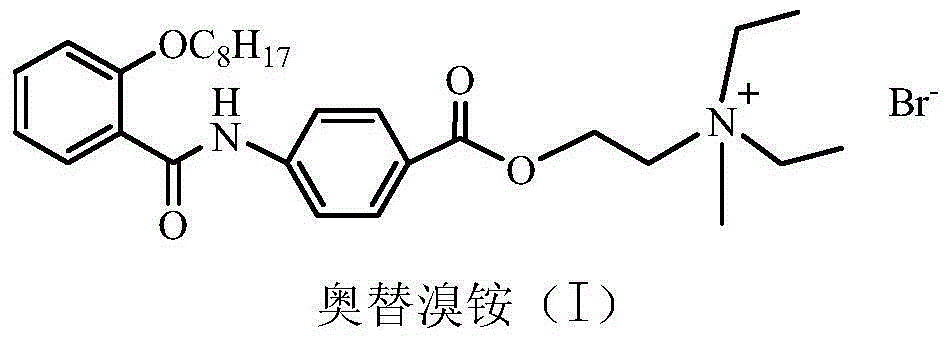
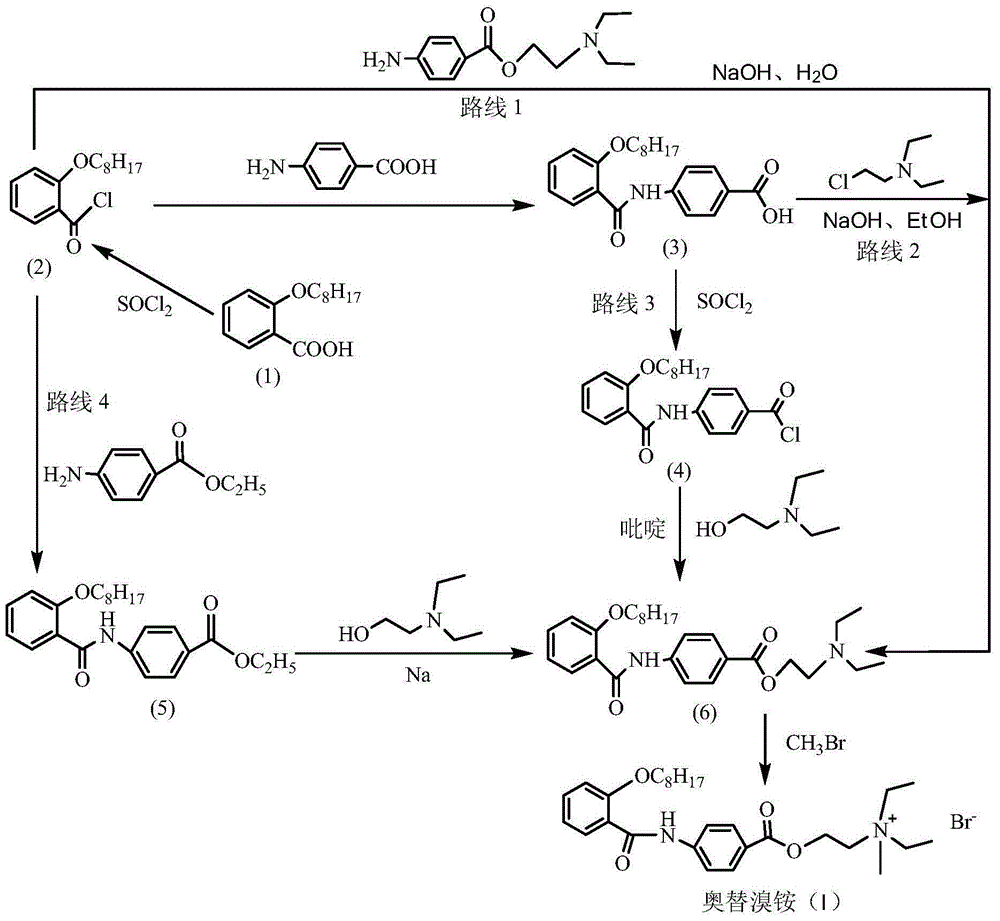
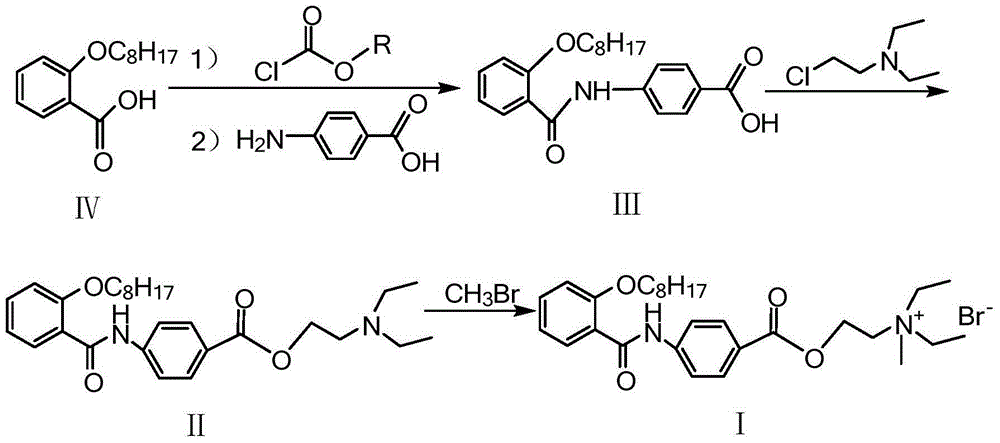
Preparation method of otilonium bromide
A technology for octilonium bromide and a compound is applied in the field of preparation of octilonium bromide, can solve problems such as being unsuitable for large-scale industrial production, unsuitable for large-scale industrial production, and difficult in industrial operation, and achieves fast and convenient preparation process, High recyclability and the effect of shortening production time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the preparation of 4-(2-octyloxybenzamido) benzoic acid
[0044]
[0045] Dissolve (50g, 0.2mol) o-octyloxybenzoic acid in 500ml of dichloromethane, add (50.6g, 0.5mol) triethylamine under stirring, cool down to 0-5°C, keep the temperature below 10°C, add dropwise (23g, 0.212mol) of ethyl chloroformate, after the dropwise reaction was completed, the temperature was kept at 0-5°C for 1h. At the end of the heat preservation, (28.6g, 0.209mol) p-aminobenzoic acid was added and reacted at 20-25°C for 18h. Add 200ml of water, stir, separate the organic layer, concentrate to dryness under reduced pressure, add 1L of water, adjust the pH to 2-3 with dilute hydrochloric acid, stir and crystallize, filter with suction, dry, and recrystallize from absolute ethanol to obtain an off-white solid product 58.5g. The yield is 80%, and the HPLC purity is greater than 98%.
[0046] end product from 1 HNMR and mass spectral characterization.
[0047] ESI-MS(m / z): 371...
Embodiment 2
[0051] Example 2: Preparation of N,N-diethyl-2-[4-(2-octyloxybenzamido)benzoyloxy]ethylamine
[0052]
[0053] (45g, 0.122mol) 4-(2-octyloxybenzamido) benzoic acid was dissolved in 600ml of acetone, and (19.8g, 0.146mol) diethylaminochloroethane and (84g, 0.609 mol) potassium carbonate, warming up to reflux for 3h. Cool to room temperature, filter, and concentrate the filtrate to dryness under reduced pressure. The concentrate is dissolved in 350 ml of petroleum ether, washed with water, dried over anhydrous sodium sulfate, filtered, cooled to crystallize, filtered with suction, and vacuum-dried at room temperature to obtain 52.6 g of a white solid product. The yield was 92%, and the HPLC purity was greater than 99%.
[0054] end product from 1 HNMR and mass spectral characterization.
[0055] ESI-MS(m / z): 470[M+H] + .
[0056] 1 HNMR (400MHz, DMSO) δ: 10.39 (s, 1H, NH), 7.92 (d, 2H, Ar-H), 7.84 (d, 2H, Ar-H), 7.65 (s, 1H, Ar-H), 7.49(s,1H,Ar-H), 7.16(s,1H,Ar-H), 7.0...
Embodiment 3
[0059] Embodiment 3: the preparation of otilonium bromide
[0060]
[0061] Add 800ml of acetone to a 1L four-neck flask, cool down to 5-10°C, and introduce (120g, 1.26mol) methyl bromide gas. After completion, add (40g, 0.085mol) N,N-diethyl-2-[4 -(2-octyloxybenzamido)benzoyloxy]ethylamine, react at 10-15°C for 12 hours, filter with suction, and dry to obtain 42 g of white crystals. The yield was 87%, and the HPLC purity was greater than 99%.
[0062] end product from 1 HNMR and mass spectral characterization.
[0063] ESI-MS (m / z): 483 [M-80] + , 484[M+H-80] + ;
[0064]
[0065] 1 HNMR (400MHz, DMSO) δ: 10.46 (s, 1H, NH), 7.98 (d, 2H, Ar-H), 7.88 (d, 2H, Ar-H), 7.64 (d, 1H, Ar-H), 7.62(d, 1H, Ar-H), 7.19(d, 1H, Ar-H), 7.07(s, 1H, Ar-H), 4.67(t, 2H, OC H 2 CH 2 N), 4.10(t,2H,OC H 2 (CH 2 ) 6 CH 3 ), 3.76(t,2H,OCH 2 C H 2 N), 3.45(q,4H,NC H 2 CH 3 ), 3.09 (s,3H,NCH 3 ), 1.74 (m,2H,OCH 2 C H 2 (CH 2 ) 5 CH 3 ), 1.38(m,2H,O(CH 2 ) 2 C H 2 )...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com