Sharpleaf galangal fruit extract capable of treating chronic kidney diseases and applications thereof
A technology for chronic kidney disease and Yizhiren, which is applied to medical preparations containing active ingredients, drug combinations, plant raw materials, etc., can solve the problems of poor patient compliance, poor patient efficacy, and long course of treatment for western medicine, and achieve convenient administration. , good renal protection, low toxicity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1, a kind of Yizhiren extract with the effect of treating chronic kidney disease, it is prepared by the following method:
[0029] Take a certain quality of nootropic fruit, break it, separate and remove the nootropic husk to get nootropic kernels, add 95% volume concentration ethanol of 10 times the volume of nootropic kernels, reflux extraction for 3 times, each time for 2 hours, collect and merge The extract is applied to a macroporous resin, first eluted with 60% ethanol to remove impurities, then eluted with 90% high-concentration ethanol, and concentrated into an extract, which is separated by a silica gel column, and the extract is separated by a volume ratio It was eluted with 19:1 petroleum ether-ethyl acetate, the eluate was concentrated, and crystallized at 4°C to obtain the extract of P. japonicus. The extract was identified as citrus ketone by modern spectroscopy, and the specific data are as follows.
[0030] Colorless crystals (4°C), ESI-MSm / z218[M+H]+; ...
Embodiment 2
[0033] 1, a kind of Yizhiren extract with the effect of treating chronic kidney disease, it is prepared by the following method:
[0034] Take a certain quality of nootropic fruit, break it, separate and remove the nootropic husk to get nootropic kernels, add 70% volume concentration ethanol of 12 times the volume of nootropic kernels, reflux extraction twice, each time for 2 hours, collect and merge The extract is applied to a macroporous resin, first eluted with a volume concentration of 50% ethanol to remove impurities, then eluted with a volume concentration of 80% high-concentration ethanol, and concentrated into an extract, which is separated by a silica gel column, and the volume ratio It is 100:1~100:10 petroleum ether-ethyl acetate gradient elution in sequence, the eluate is concentrated, and crystallized at 4°C to obtain the extract of P. japonicus. The extract is identified as narone by modern spectroscopy, which is the same as in Example 1 by modern spectroscopy. ...
Embodiment 3
[0035] Embodiment 3 treats the pharmacodynamics test of chronic kidney disease
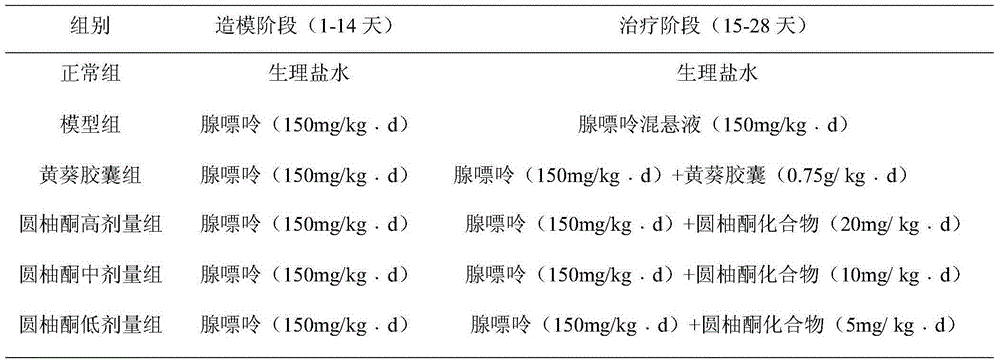
[0036] 1. Drug effect research on adenine-induced kidney injury in rats: 48 mice were randomly divided into 6 groups, i.e. normal group, model group, positive drug group, and narone prepared in Example 1 of the present invention Compound high, medium and low dosage groups, 8 rats in each group, were administered according to Table 1.
[0037] Table 1 Dosing regimen of Yizhiren extract for drug efficacy study on adenine-induced kidney injury in rats
[0038]
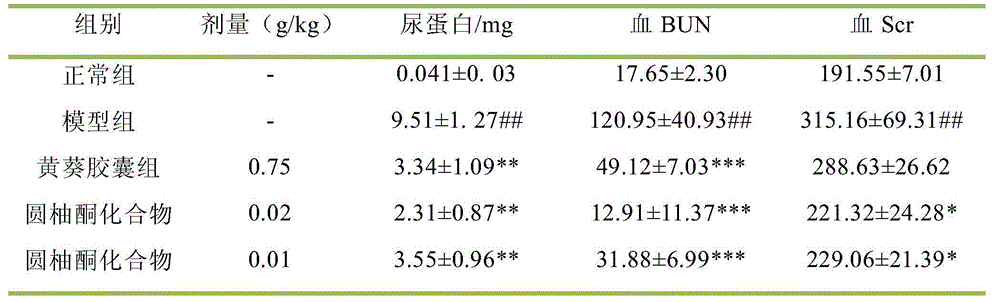
[0039]14 days after administration. Rats in each group were placed in metabolic cages, fasted without water, collected 24-hour urine, and measured 24-hour urine volume and urine protein (UP 24h ). After fasting for 6 hours, the rats were anesthetized with ether, blood was collected from the abdominal aorta, and the serum was separated to measure blood urea nitrogen (BUN), serum creatinine (Scr), serum superoxide dismutase (SOD) and malo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com