Sodium prasterone sulfate sustained-release tablet and preparation method thereof
A technology of prasterone sulfate sodium and slow-release tablets, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve problems that need to be deepened
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] According to the second aspect of the present invention, the present invention also proposes a method for preparing the aforementioned prasterone sulfate sodium sustained-release tablet. The preparation method of the prasterone sulfate sodium sustained-release tablet according to specific embodiments of the present invention comprises:
[0050] (1) Prasterone Sulfate Sodium, skeleton material and filler are mixed;
[0051] (2) Add binder to the mixture obtained in step (1), and make soft material successively, granulate, and dry;
[0052] (3) Sizing the granules obtained through the drying, adding a lubricant, mixing evenly, and compressing into tablets to obtain the prasterone sulfate sodium sustained-release tablets.
[0053] Thus, the present invention has prepared Prasterone Sulfate Sodium Sustained-release Tablets by adopting the above-mentioned wet granulation tableting technology. According to a specific embodiment of the present invention, the prasterone sulfa...
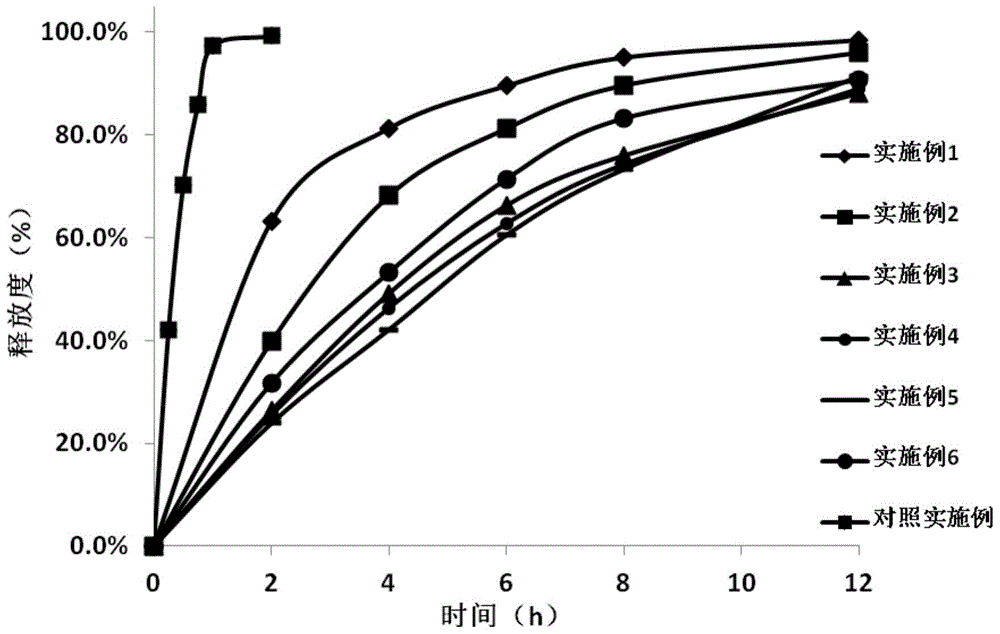
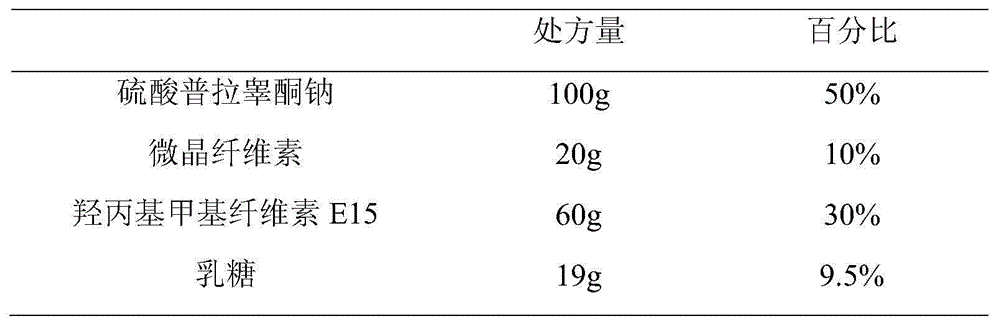
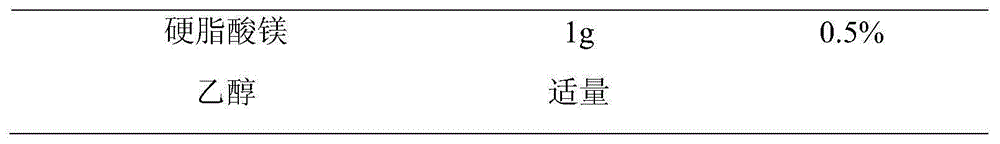
Embodiment 1
[0066]
[0067]
[0068] Preparation:
[0069] (1) Pass through an 80-mesh sieve after the raw and auxiliary materials are pulverized respectively, and set aside;
[0070] (2) Take by weighing prasterone sulfate sodium and hydroxypropyl methylcellulose E15 of prescription quantity and mix for 3 minutes, add microcrystalline cellulose and lactose of prescription quantity and mix for 2 minutes;
[0071] (3) Add an appropriate amount of ethanol to make a soft material, pass through a 24-mesh sieve to granulate, and dry in an oven at 60 degrees Celsius for 1 hour;
[0072] (4) Cross a 26-mesh sieve for granulation, add magnesium stearate in the prescribed amount, mix evenly, and compress the tablet, and the pressure is controlled at 6-7 kg during tablet compression.
Embodiment 2
[0074] (Specification 100mg, 1000 tablets):
[0075]
[0076] Preparation:
[0077] (1) Pass through a 60-mesh sieve after the raw and auxiliary materials are pulverized respectively, and set aside;
[0078] (2) Take by weighing prasterone sulfate sodium and hydroxypropyl methylcellulose K4M of prescription quantity and mix for 3 minutes, add microcrystalline cellulose and lactose of prescription quantity and mix for 2 minutes;
[0079] (3) adding an appropriate amount of ethanol to make a soft material, passing through an 18-mesh sieve to granulate, and drying in an oven at 40 degrees Celsius for 2 hours;
[0080] (4) Cross a 26-mesh sieve for granulation, add magnesium stearate in the prescribed amount, mix evenly, and compress the tablet, and the pressure is controlled at 7-8 kg during tablet compression.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com