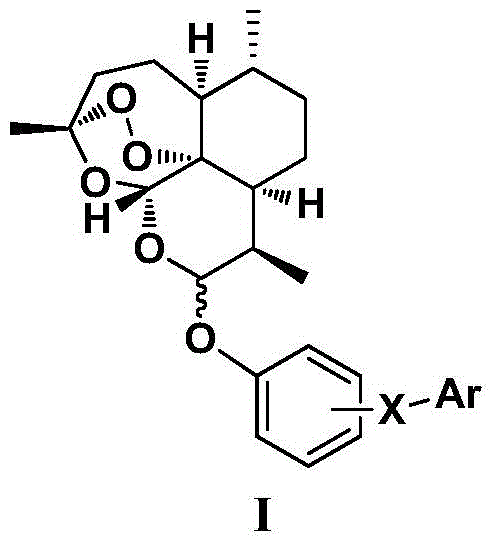
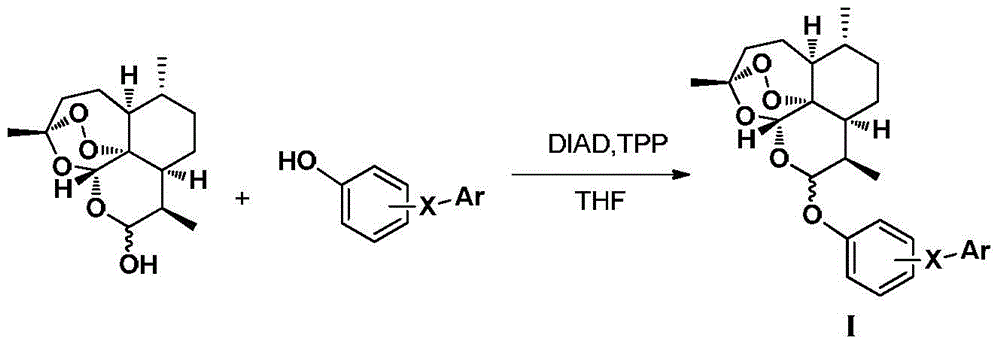
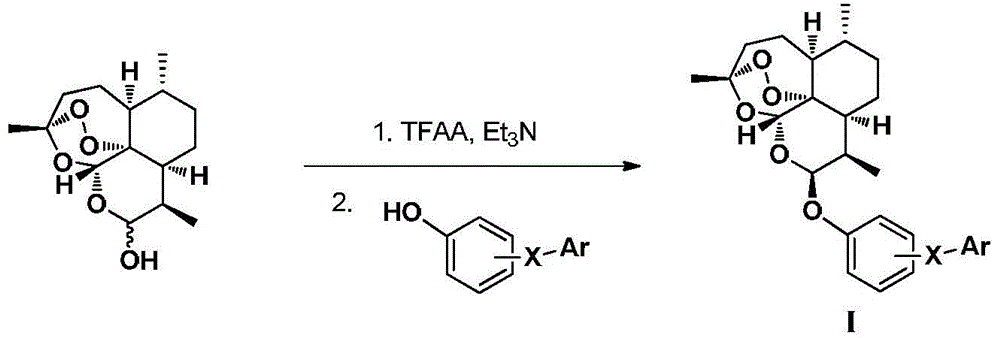
Dihydroartemisinin phenyl ether derivatives and their application
A dihydroartemisinin and phenyl technology, applied in the field of medicine, can solve problems such as ineffectiveness, induced drug resistance, and small therapeutic index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0105] The examples are intended to illustrate, not limit, the scope of the invention. All technologies realized based on the above contents of the present invention belong to the scope of the present invention.
[0106] The nuclear magnetic resonance spectrum of the compound was measured by Bruker ARX-300, and the mass spectrometer was measured by the WaterQuattro micro API triple quadrupole tandem mass spectrometer of Water Company of the United States; after the reaction of the compound containing the dihydroartemisinin mother nucleus, the treatment water was purified water or distilled water, and other Reagents are commercially available products of analytical or chemical purity.
Embodiment 1
[0107] Example 1: Preparation of 10-O-[4-[5-(4-chlorophenyl)-1H-3-pyrazolyl]phenyl]-(10S)-dihydroartemisinin
[0108] Step A: Preparation of 1-(4-hydroxyphenyl)-3-(4-chlorophenyl)-2-propen-1-one
[0109]
[0110] Dissolve 4 g (0.1 mol) of NaOH in 30 ml of anhydrous methanol and stir in an ice bath. Dissolve 1.54g (0.01mol) of p-chlorobenzaldehyde and 1.36g (0.01mol) of p-hydroxyacetophenone in 30mL of anhydrous methanol, and add dropwise to the above solution. After the dropwise addition, the temperature is raised to room temperature, and the reaction is continued for 2 hours. The methanol was distilled off under reduced pressure, and 50 mL of H 2 O, add 5 mol / L hydrochloric acid dropwise, adjust the pH to 8, and filter with suction to obtain a light yellow solid, which is dried under infrared light.
[0111] Step B: Preparation of [1-acetyl-4,5-dihydro-3-(4-hydroxyphenyl)-5-(4-chlorophenyl)]pyrazole
[0112]
[0113] Dissolve 2.58g (0.01mol) of 1-(4-hydroxyphenyl)-3-...
Embodiment 2
[0128] Example 2: Preparation of 10-O-[4-[5-(4-chlorophenyl)-1H-3-pyrazolyl]phenyl]-(10R)-dihydroartemisinin
[0129] According to the preparation method of Example 1, the title compound can be obtained simultaneously.
[0130] LC-MS(m / z):537.2[M+H] + ,559.1[M+Na] +
[0131] 1 H-NMR(DMSO,δ(ppm)):13.28(br,1H,1H-H),7.86(m,2H,Ar-H),7.75(m,2H,Ar-H),7.50(m,2H ,Ar-H),7.19(m,2H,Ar-H),7.14(s,1H,P4-H),5.74(s,1H,H-12),5.30(d,1H,J=9.3Hz, H-10),2.51(m,1H,H-9),2.20(m,1H,H-4),1.35(s,3H,H-14),0.92(m,6H,H-15andH-16) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com