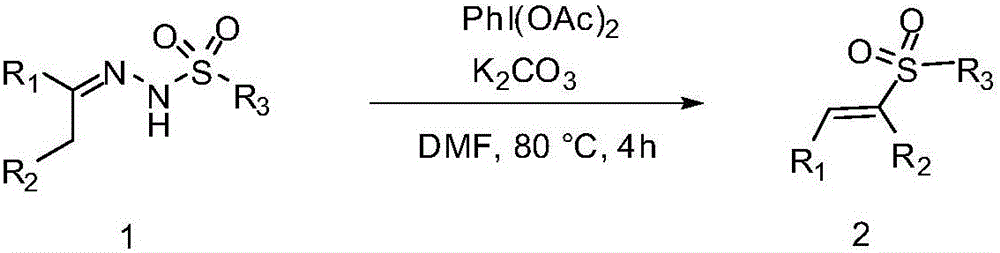
A kind of method of synthesizing e alkenyl sulfone compound
A technology of compound and alkenyl sulfone, which is applied in the field of synthesizing E-alkenyl sulfone compound by catalytic degradation of benzenesulfonyl hydrazone with iodobenzene diacetate, can solve the problems such as not having environmental friendliness, and achieve low reaction toxicity and high reaction efficiency. The effect of mild conditions and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
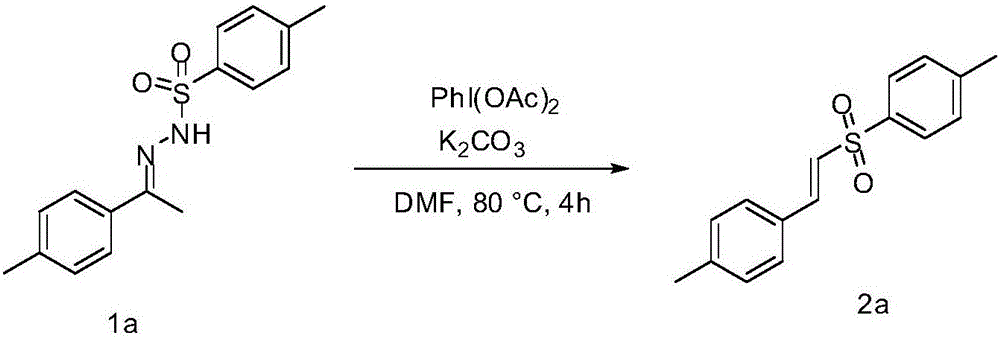
Embodiment 1
[0023] Implementation Example 1: Add 64mg (0.2mmol) benzenesulfonylhydrazone compound 1a, 71mg (0.22mmol) PhI(OAc) in a round bottom flask 2 and 28 mg (0.2 mmol) K 2 CO 3 , adding 2mL of DMF, in an oil bath at 80°C, reacting with magnetic stirring for 4h, and monitoring the progress of the reaction by TLC. After the reaction, extract with ethyl acetate and separate by column chromatography [petroleum ether (60~90°C) / ethyl acetate=15 / 1] to obtain (E)-1-methyl-4-(4-methylbenzene Vinyl)sulfonylbenzene 2a, white solid 45mg, yield 83%, Mp: 92-94℃. 1 H NMR (400MHz, CDCl 3 )δ7.87(d,J=6.4Hz,2H,ArH)7.68(d,J=12.0Hz,1H,CH),7.42(m,4H,ArH),7.24–7.22(m,2H,ArH), 6.84(d,J=12.0Hz,1H,CH),2.47(s,3H,CH 3 ),2.41(s,3H,CH 3 ); 13 C NMR (100MHz, CDCl 3 )δ148.11, 145.09, 131.55, 130.42, 129.17, 129.14, 128.09, 115.86, 115.68, 22.04, 21.70. MS (ESI): m / z = 294.9 [M + Na] + ,567.0[2M+Na] + The reaction principle of implementation example 1 is as follows:
[0024]
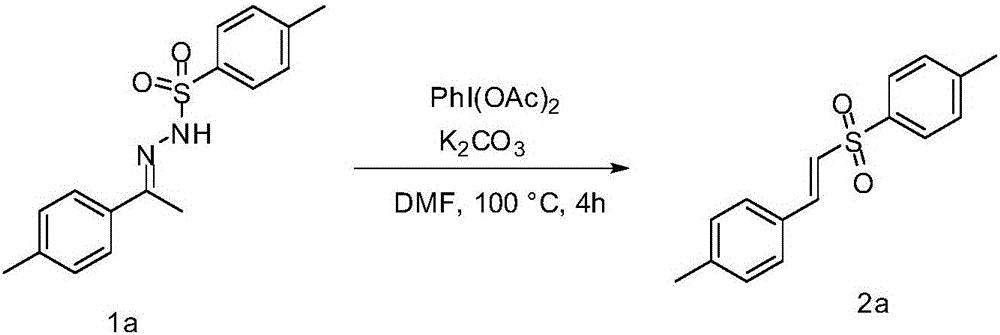
Embodiment 2
[0025] Implementation Example 2: Add 64mg (0.2mmol) benzenesulfonylhydrazone compound 1a, 71mg (0.22mmol) PhI(OAc) in a round bottom flask 2 and 28 mg (0.2 mmol) K 2 CO 3 , adding 2mL of DMF, under the condition of 100°C in an oil bath, magnetically stirred for 4h, and monitored the progress of the reaction by TLC. After the reaction, extract with ethyl acetate and separate by column chromatography [petroleum ether (60~90°C) / ethyl acetate=15 / 1] to obtain (E)-1-methyl-4-(4-methylbenzene Vinyl)sulfonylbenzene 2a, white solid 38mg, yield is 70%, the reaction principle of implementation example 2 is as follows:
[0026]
Embodiment 3
[0027] Implementation Example 3: Add 64mg (0.2mmol) benzenesulfonylhydrazone compound 1a, 71mg (0.22mmol) PhI(OAc) in a round bottom flask 2 and 28 mg (0.2 mmol) K 2 CO 3 , adding 2mL of DMF, in an oil bath at 80°C, reacting with magnetic stirring for 2h, and monitoring the progress of the reaction by TLC. After the reaction, extract with ethyl acetate and separate by column chromatography [petroleum ether (60~90°C) / ethyl acetate=15 / 1] to obtain (E)-1-methyl-4-(4-methylbenzene Vinyl)sulfonylbenzene 2a, white solid 41mg, yield is 75%, the reaction principle of implementation example 3 is as follows:
[0028]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com