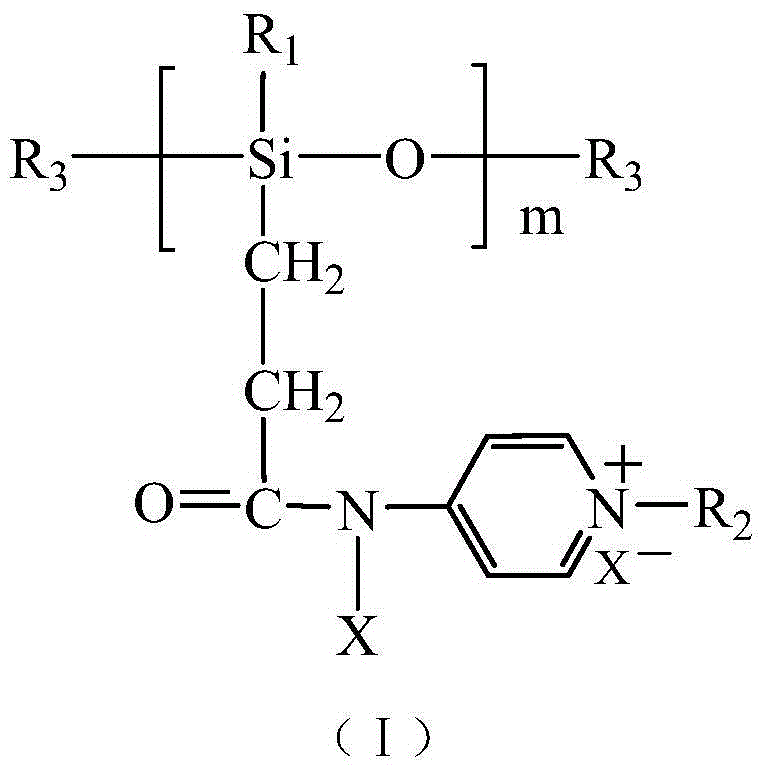
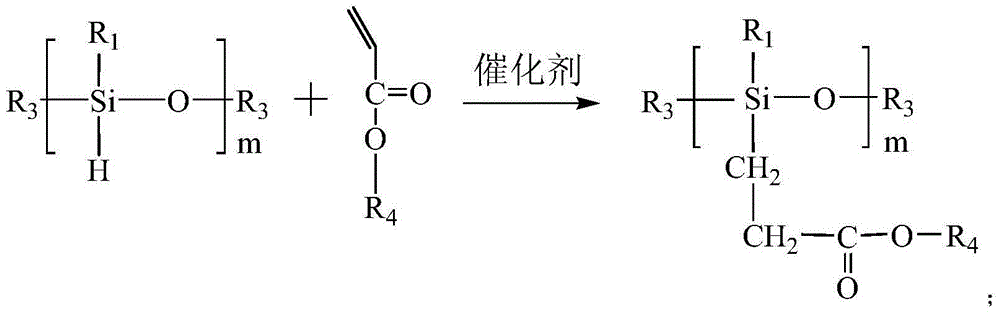
Halamine-pyridine salt double-function group polysiloxane bactericide, and preparation method and application thereof
A technology of halamine pyridine salt and bactericide, which is applied in the field of polymer synthesis, can solve the problems of limited bactericidal ability, and achieve the effect of perfect protection, low application risk and strong killing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Add 60 parts of R 1 =CH 3 , R 3 =H perhydrogen silicone oil, 0.005 parts of chloroplatinic acid, 300 parts of toluene, and 153 parts of tert-butyl acrylate. The system uses dry nitrogen to remove water and air, reacts at 110°C for 20 to 24 hours, then distills off toluene under reduced pressure, adds 140 parts of trifluoroacetic acid, and conducts hydrolysis reaction at 20°C for 1 to 1.2 hours to dissolve lipid bonds. Hydrolyzed to carboxyl. Then add 118 parts of 4-aminopyridine to the resulting carboxyl-containing silicone oil, use toluene as a solvent, and add 0.01 part of 2-chloro-4,6-dimethoxy-1,3,5-triazine as a catalyst. After 8-9 hours of reaction, the carboxyl group in the silicone oil reacts with the amino group in the 4-aminopyridine to form an amide bond. Then, 200 parts of 1-bromo-n-hexane was added dropwise at room temperature, and reacted for 20 to 24 hours, after which the solvent and unreacted 1-bromo-n-hexane were distilled off under reduced pressur...
Embodiment 2
[0065] Add 25 parts of R 1 =CH 2 CH 3 , R 3 =H hydrogen silicone oil, 0.003 parts of chloroplatinic acid, 100 parts of toluene, and 65 parts of tert-butyl acrylate. The system uses dry nitrogen to remove water and air, reacts at 110° C. for 24 hours, then distills off toluene under reduced pressure, adds 60 parts of trifluoroacetic acid and carries out hydrolysis reaction at room temperature for 1 hour, and hydrolyzes lipid bonds into carboxyl groups. Then add 50 parts of toluene, 50 parts of 4-aminopyridine and 0.02 parts of dicyclohexylcarbodiimide to the three-necked flask, and raise the temperature to 60°C to react the carboxyl group with the amino group to form an amide bond. Then, 180 parts of 1-chloro-n-decane were added with a dropping funnel at room temperature and reacted for 24 hours, after which the toluene solvent and unreacted 1-chloro-n-decane were distilled off under reduced pressure. Finally, 100 parts of n-propanol and 100 parts of tert-butyl hypochlorite...
Embodiment 3
[0067] Add 50 parts of R 1 =CH 2 CH 3 , R 3 =C(CH 3 ) 3 Hydrogen-containing silicone oil, 0.01 parts of methyl vinyl siloxane coordination platinum, 150 parts of toluene, and 120 parts of methyl acrylate. The system used dry nitrogen to remove water and air, reacted at 100°C for 24 hours, then distilled off toluene under reduced pressure, added 125 parts of trifluoroacetic acid and carried out hydrolysis reaction at room temperature for 6 hours, and hydrolyzed the lipid bonds into carboxyl groups. Then add 100 parts of 4-aminopyridine, 80 parts of toluene and 0.03 parts of 2-chloro-4,6-dimethoxy-1,3,5-triazine to the resulting carboxyl-containing silicone oil, and react at 30°C for 8 hours , the carboxyl group in silicone oil reacts with the amino group in 4-aminopyridine to form an amide bond. Then, 200 parts of 1-bromo-n-hexane was added dropwise at room temperature, and reacted for 24 hours, after which the solvent and unreacted 1-bromo-n-hexane were distilled off und...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com