Recombinant plasmid of specific ligand of BK channel and recombinant expression method thereof
An expression method and specific technology, applied in the field of recombinant plasmids of specific ligands of BK channels and their recombinant expression fields, can solve the problems of low yield and activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: Construction of pGEX-4T-3-Martentoxin plasmid
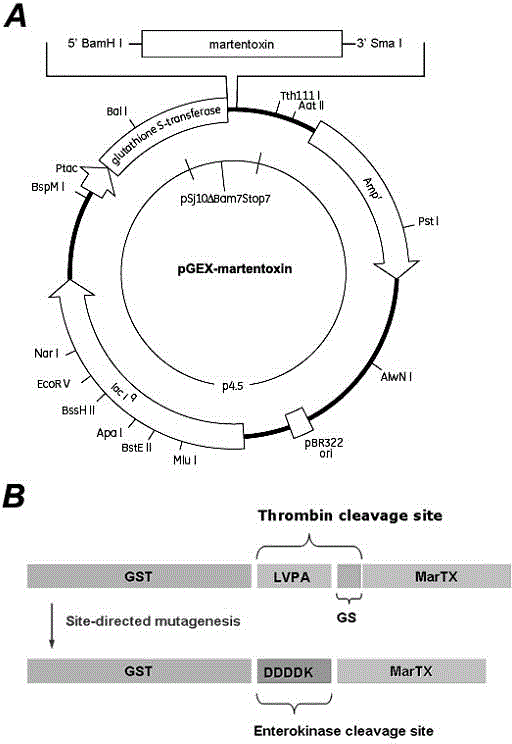
[0043] Based on the cDNA sequence of MarTX (GenBank No. AF534113.1), two pairs of primers were designed to amplify the coding sequence of MarTX and introduce the restriction site on the vector pGEX-4T-3. MarTX-forward primer (5'-TTC GGATCC TTTGGACTCATAGA-3'), which contains a BamH I restriction site (underlined); MarTX-reverse primer (5'-CTT CCCGGG TTAATCAGTAGCAT-3') contains a Sma I restriction site (underlined). The cDNA sequence of MarTX was inserted between the two restriction sites of BamH I and Sma I of pGEX-4T-3, thereby obtaining the complete expression plasmid pGEX-4T-3-MarTX, such as figure 1 -A shown.
[0044] In this expression system, 6 redundant base pairs appear between the thrombin cleavage site and the cDNA sequence of MarTX. This will lead to two redundant amino acid residues (GS, figure 1 B). Excess base pairs were removed by point mutagenesis as described using the KOD Mutati...
Embodiment 2
[0045] Example 2: In vitro expression and purification of rMarTX
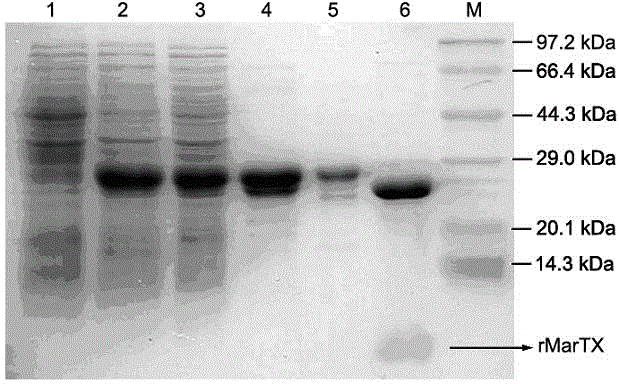
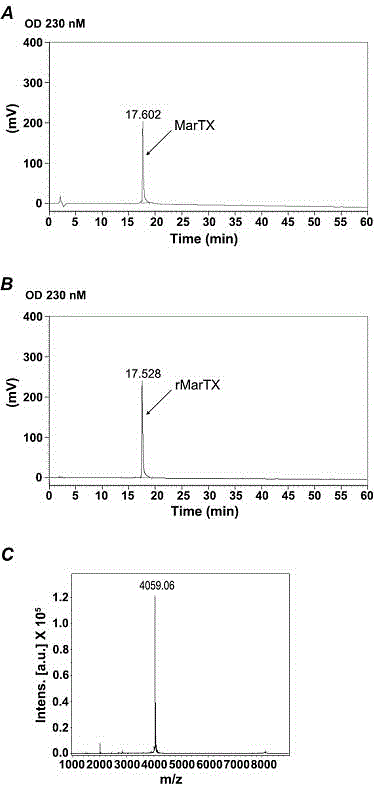
[0046] The expression plasmid pGEX-4T-3-MarTX was transformed into Escherichia coli strain BL21 (DE3), and the strain cells were grown in Luria-Bertani (LB) medium (volume 1 L) containing 0.1 mg / mL ampicillin. The temperature is 37°C. When the bacteria solution OD 600When the value reached 0.4-0.6, Isopropyl-β-d-thiogalactoside (IPTG) was added at a final concentration of 0.5 mM to induce the expression of the toxin protein. E. coli cells were grown continuously at 28 °C for 4 hours. After culturing, cells were collected by centrifugation at 5000 g for 10 min, and resuspended in 70 mL of 1× phosphate buffered saline (PBS, 137 mM NaCl, 4.3 mM Na2HPO4, 2.7 mM KCl, 1.4 mM KH2PO4, pH 7.4). Cells were disrupted in an ice bath and sonicated (4 bursts / min) for 20 min. The lysate was centrifuged at 12,000 g for 15 min at 4 °C. The supernatant was filtered with a 0.45 filter membrane, and passed through an Econo-ch...
example 3
[0050] Example 3: Electrophysiological detection of recombinant rMarTX
[0051] 3.1 Solution preparation for electrophysiological experiments
[0052] In a patch clamp experiment, the extracellular fluid of BK channel (mM): NaCl 135, KCl 5, MgCl 2 1.2, CdCl 2 2.5, HEPES 5, glucose 10 (adjust pH to 7.4 with NaOH); electrode inner solution (mM): NaCl 10, KCl 117, MgSO 4 2, HEPES 10, MgATP 2, EGTA 1 (adjust pH to 7.2 with KOH).
[0053] Extracellular fluid of mKv1.3 and hKv3.1a channels (mM): NaCl 135, KCl 5, MgCl2 1, CaCl2 1.8, HEPES 10, glucose 10 (adjust pH to 7.4 by NaOH); extracellular fluid of hKv4.2 channel (mM): NaCl 125, KCl 2, MgCl2 1, glucose 10, HEPES 10, TEA 20 (adjust pH to 7.4 by NaOH). Electrode inner solution of mKv1.3, hKv3.1a and hKv4.2 (mM): KCl 130, MgCl2 0.5, MgATP 2, EGTA 10, HEPES 10 (adjust pH to 7.3 by KOH).
[0054] 3.2 Transient transfection
[0055] BK channels, mKv1.3, hKv3.1a and hKv4.2 channels were all expressed in HEK293T cells by transi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com