Preparation method of recombinant human growth hormone and preparation method of PEGylation modification substance
A technology for the modification of human growth hormone, which is applied in the field of preparation of polyethylene glycol modifications, can solve the problems of foreign protein interference, complex process steps, low activity of human growth hormone, etc., and achieve rapid and efficient separation and high application value Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The recombinant human growth hormone used in this example has a purity greater than 95% and maintains high biological activity.
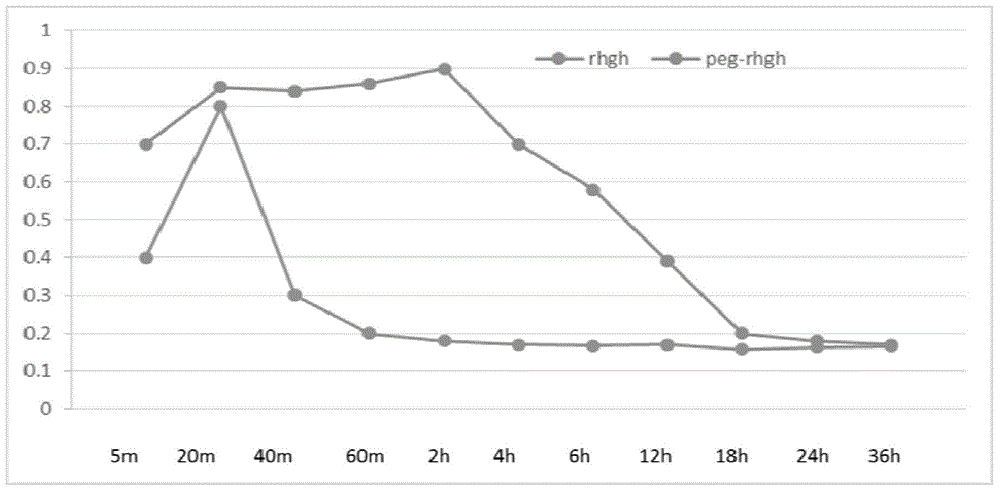
[0055] Dissolve recombinant human growth hormone (rhGH) in 100Mm phosphate buffer (PH10), add PEG amber according to the ratio of recombinant human growth hormone (rhGH): polyethylene glycol (PEG) = 1:3 (W / W) Imido-succinamide ester (mPEG-SS), stirred and dissolved, reacted at 4°C for 2 hours.
Embodiment 2
[0057] The recombinant human growth hormone used in this example has a purity greater than 95% and maintains high biological activity.
[0058] Dissolve recombinant human growth hormone (rhGH) in 100Mm phosphate buffer (PH8), add PEG amber according to the ratio of recombinant human growth hormone (rhGH): polyethylene glycol (PEG) = 1:6 (W / W) Imido-succinamide ester (mPEG-SS), stirred and dissolved, reacted at 4°C for 2 hours.
Embodiment 3
[0061] The product obtained by reacting recombinant human growth hormone (rhGH) with polyethylene glycol was dissolved in 100Mm phosphate buffer (PH8) and dialyzed, after being adsorbed by anion exchange resin or cation exchange resin, it was eluted with salt, and polyethylene glycol was collected. The alcoholized recombinant human growth hormone drug is concentrated and purified by molecular sieve gel chromatography. The purity of the obtained polyethylene glycol recombinant human growth hormone drug can be greater than 95%, and the product is obtained after small filtration and freeze-drying .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com