Sildenafil citrate composition tablet as medicine for treating urological diseases
A technology of sildenafil citrate and urology, applied in the field of medicine, can solve the problems of large influence on drug stability, difficult preparation of preparations, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the preparation of sildenafil citrate crystal
[0028] (1) Add the solid sildenafil citrate to a mixed solvent of 2,4-lutidine and ether at 45°C, the volume of the mixed solvent is 10 times the weight of sildenafil citrate, 2, The volume ratio of 4-lutidine to ether is 5:1.5;
[0029] (2) Under the condition of controlling the temperature at 45°C, add a mixed solvent of ethanol and methyl salicylate to the solution obtained in step (1), the volume of the mixed solvent is 12 times the weight of sildenafil citrate, The volume ratio of ethanol and methyl salicylate is 3:1;
[0030] (3) After adding the mixed solvent of ethanol and methyl salicylate, cool down to -10-°C at a rate of 6.5°C / min, and stand at -10-°C for 5 hours to precipitate crystals, filter, wash, and vacuum dry The sildenafil citrate compound is obtained.
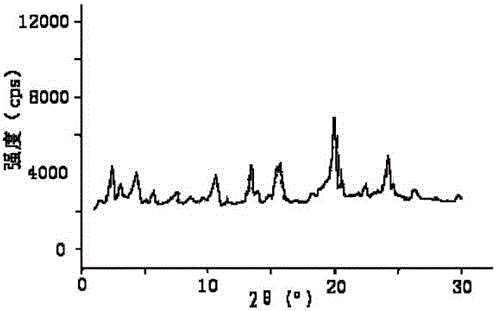
[0031] The prepared sildenafil citrate crystal uses Cu-Kα ray to measure the X-ray powder diffraction pattern obtained as figure 1 sho...
Embodiment 2
[0032] Embodiment 2: the preparation of sildenafil citrate composition tablet:
[0033] Prescription: in parts by weight as shown in Table 1
[0034] Table 1 Prescription of sildenafil citrate composition
[0035]
[0036] (1) Processing of raw and auxiliary materials: Sodium carbonate, dextrin, sucrose, and sildenafil citrate are passed through a 100-mesh sieve with a vibrating sieve;
[0037] (2) Weighing: Weigh each raw and auxiliary material according to the prescription amount;
[0038] (3) Preparation of adhesive: Dissolve the prescribed amount of povidone K30 in purified water to make an aqueous solution of povidone k30;
[0039] (4) Granulation: add internally added raw and auxiliary materials sildenafil citrate, sodium carbonate, starch, dextrin, sucrose, and crospovidone into the high-speed mixing granulator, and turn on the stirring motor at low speed (I speed) Mix for 10 minutes, add povidone K30 aqueous solution, wet mix at low speed (I speed) for 90-120 sec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com