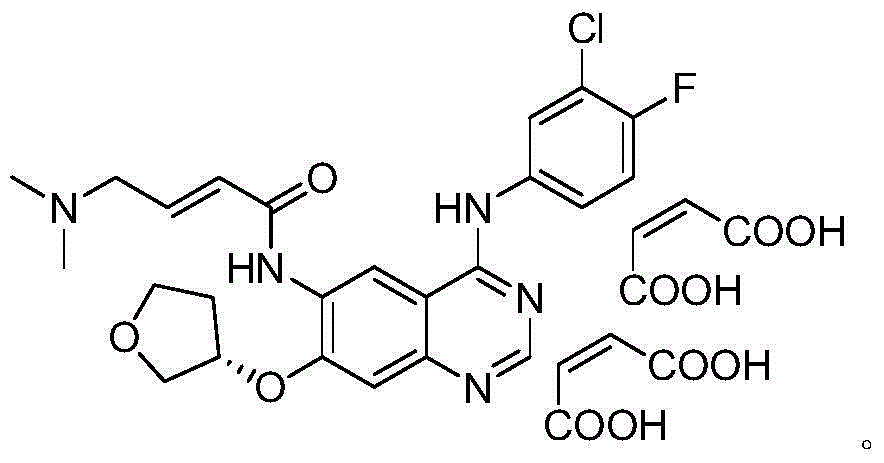
Crystal form of afatinib di-meleate and method for preparing crystal form
A crystallization and form technology, applied in the fields of organic chemistry, drug combination, antitumor drugs, etc., can solve the problems of easy sticking, easy splitting or lamination, low bulk density, etc., and achieves low production cost, excellent chemical purity, and operation. simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
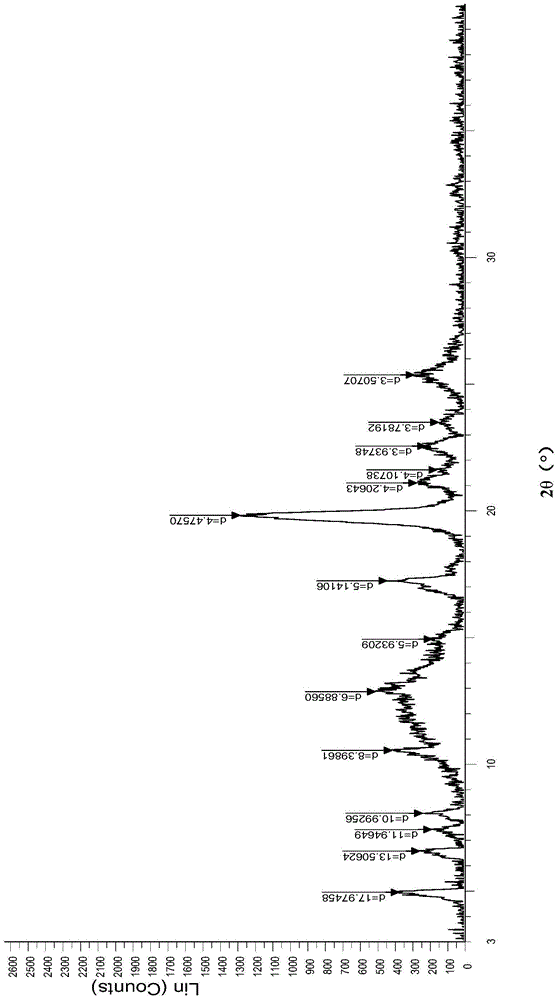
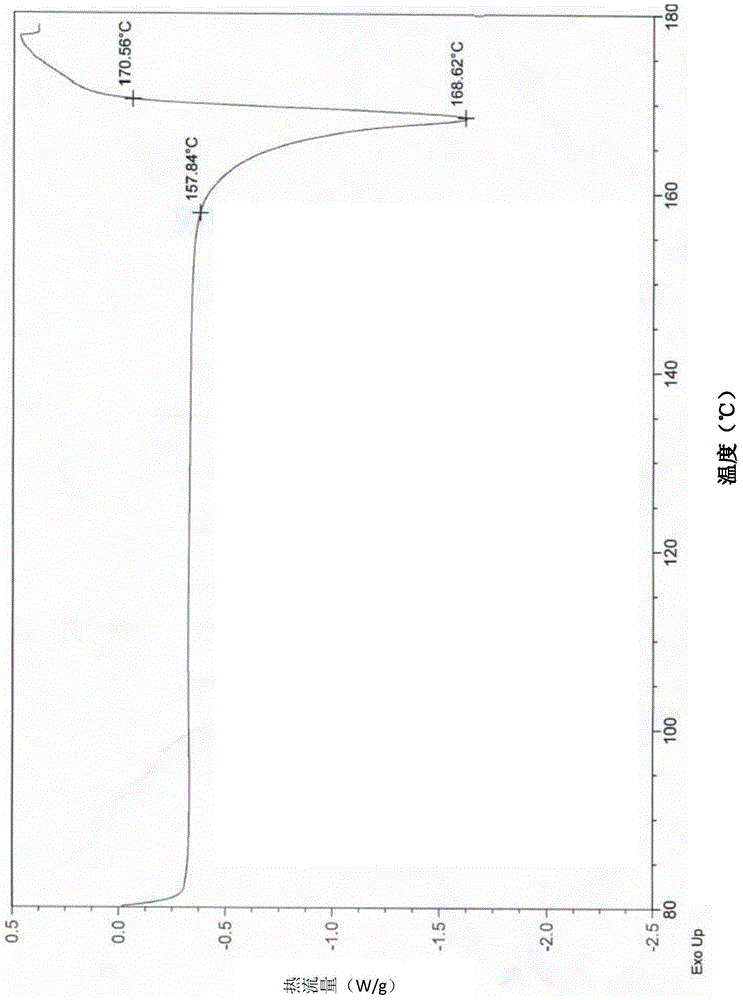
[0022] Example 1 Preparation of Afatinib Dimaleate Crystal Form H
[0023] Dissolve 4g (8.23mmol) of afatinib base in 40ml of ethyl acetate, heat to 65°C to dissolve, then slowly add 2g (17.3mmol) of maleic acid in ethyl acetate (5ml) solution dropwise to the above solution, After the dropwise addition, it was naturally cooled to room temperature, then rapidly cooled to 0°C, stirred for 2 hours, a large amount of solids were precipitated, and the off-white powder was obtained by suction filtration, dried in vacuum at 40°C for 4 hours, and the purity (HPLC: 99.55%) was obtained.
Embodiment 2
[0024] Example 2 Preparation of Afatinib Dimaleate Crystal Form H
[0025] Dissolve 10g (20.58mmol) of afatinib base in 100ml of ethyl acetate, heat to 65°C to dissolve, then slowly add 5g (43.08mmol) of maleic acid in ethyl acetate (10ml) solution dropwise to the above solution, After the dropwise addition, it was naturally cooled to room temperature, then rapidly cooled to 0°C, stirred for 2 hours, a large amount of solids were precipitated, filtered by suction to obtain off-white powder, dried in vacuum at 40°C for 4 hours, and the purity was high (HPLC: 99.74%).
Embodiment 3
[0026] Example 3 Preparation of Afatinib Dimaleate Form H
[0027] Dissolve 50g (102.9mmol) of afatinib base in 500ml of ethyl acetate, heat to 65°C to dissolve, then slowly add 24.5g (211.0mmol) of maleic acid in ethyl acetate (50ml) dropwise to the above solution After the dropwise addition, it was naturally lowered to room temperature and then rapidly cooled to 0° C., stirred for 2 hours, a large amount of solids were precipitated, filtered by suction to obtain off-white powder, dried in vacuum at 40° C. for 6 hours, and the purity was high (HPLC: 99.77%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com