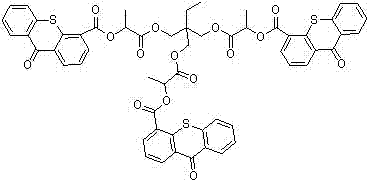
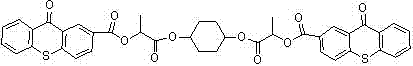
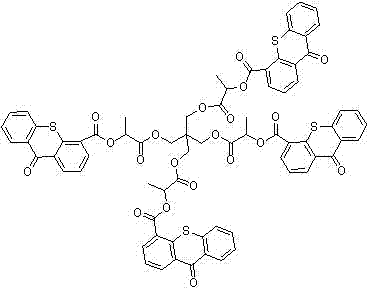
A kind of thioxanthone carboxylate photoinitiator and preparation method thereof
A technology of thioxanthone carboxylate and thioxanthone formyloxymethyl propionate, which is applied in organic chemistry and other fields, can solve the problems of low volatility, large synthesis pollution, and high cost, and achieve mild reaction conditions and simple operation , low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Methyl 2-(thioxanthone-4-formyloxy)propionate
[0050] In a 250ml four-neck flask equipped with mechanical stirring, add 25.0g thioxanthone-4-formyl chloride, 125ml chlorobenzene, 11.1g triethylamine, 10.4g methyl lactate, heat to 60~70℃, reaction 5. After hours, suction filtration, the filtrate was cooled to 5~10℃, stirred and crystallized for 2 hours, and then filtered to obtain a crude product. The crude product was recrystallized from chlorobenzene. After drying, 27.5g of pale yellow flake crystals were obtained. The yield was 88%, and the content was ≥98.5%. . 1 H NMR (DMSO) δ: 8.82 (m, 1H), 8.56 (m, 1H), 8.43 (m, 1H), 7.90(d, 1H), 7.75 (m, 2H), 7.61 (m, 1H), 5.37 (m, 1H), 3.76 (s, 3H), 1.62 (d, 3H).
Embodiment 2
[0051] Example 2: Methyl 2-(thioxanthone-4-formyloxy)propionate
[0052] In a 250ml four-neck flask equipped with mechanical stirring, add 25.0g thioxanthone-4-formyl chloride, 125ml chlorobenzene, 17.6g diisopropylethylamine, 18.9g methyl lactate, and heat to 80~90℃, React for 4 hours, filter with suction, cool the filtrate to 5~10℃, stir and crystallize for 2 hours, and then filter with suction to obtain a crude product. The crude product is recrystallized with chlorobenzene. After drying, 28.3g of yellow flake crystals are obtained. The yield is 91%, and the content is ≥98.5. %.
Embodiment 3
[0053] Example 3: Methyl 2-(thioxanthone-2-formyloxy)propionate
[0054] In a 250ml four-necked flask equipped with mechanical stirring, add 25.0g thioxanthone-2-formyl chloride, 125ml chlorobenzene, 11.1g triethylamine, 10.4g methyl lactate, heat to 60~70℃, reaction 5. After hours, suction filtration, the filtrate was cooled to 5~10℃, stirred and crystallized for 2 hours, and then filtered to obtain a crude product. The crude product was recrystallized from chlorobenzene. After drying, 29.0g of pale yellow flake crystals were obtained. The yield was 93% and the content was ≥98.5%. . 1 H NMR (DMSO) δ: 8.45 (d, 1H), 8.14 (m, 1H), 7.99 (m, 1H), 7.83(d, 1H), 7.62 (m, 2H), 7.42 (m, 1H), 5.53 (m, 1H), 3.88 (s, 3H), 1.55 (d, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com