Novel method for preparing recombinant exenatide or derivative thereof
A technology for exenatide and its derivatives, which is applied in the field of preparing exenatide or its derivatives with natural N-terminals, which can solve the problems of low ratio of small molecule target protein, affecting the final yield of small molecule target protein, etc. , to achieve the effect of enhancing stability, increasing the final expression level, and avoiding degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Design and Cloning of Hirudin III (HV3) Fusion Tag and Target Polypeptide Exendin (Exendin-4) Fusion Gene
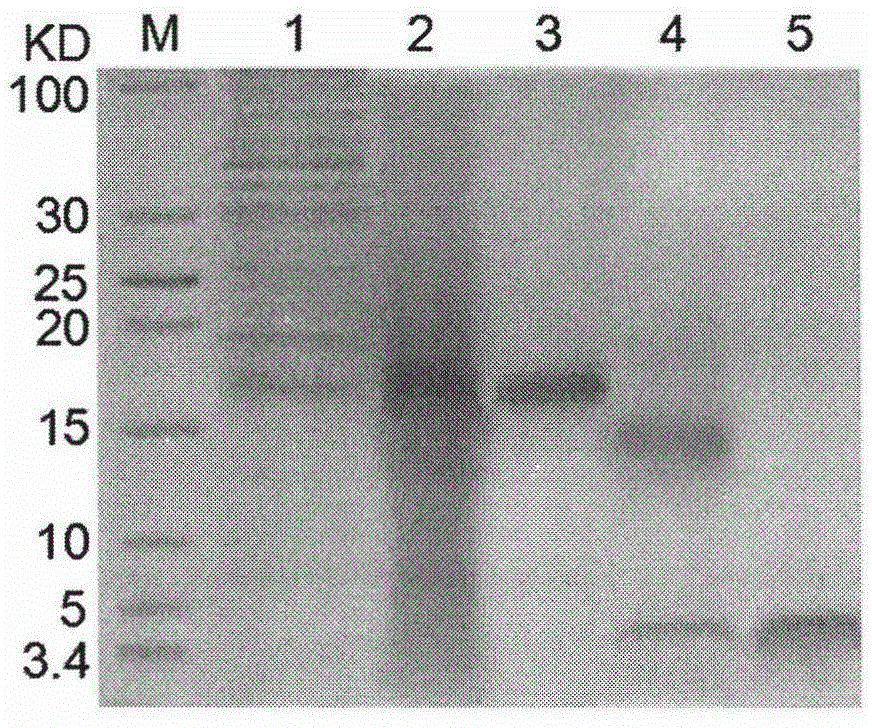
[0022]Hirudin III (HV3)-Exendin-4 fusion protein includes the following parts (from N-terminal to C-terminal): (1) 66 amino acid residues of hirudin III (HV3); (2) connecting peptide GGGGSENLYFQ↓H (where ENLYFQH is the TEV enzyme recognition cleavage sequence, the arrow indicates the cleavage site); (3) TEV enzyme cleavage site (indicated by the arrow) is followed by 39 amino acid residues of Exendin-4 base. On this basis, the above-mentioned fusion protein encoding gene was designed and synthesized according to E.coli preferred codons (see figure 1 ).
Embodiment 2
[0023] Example 2 Construction of Hirudin III (HV3)-Exendin (Exendin-4) Fusion Protein Expression Strain
[0024] The fusion gene described in Example 1 was digested with Nhe I and Hind III and then subcloned into the expression vector pTASH (Tan S, Wu W, Liu J, Protein Expr Purif2002, 25, 430-436.) site, the resulting recombinant expression vector was named pTASHE (see figure 2 ), and sequenced to verify its correctness. with CaCl 2 The recombinant expression plasmid pTASHE was transformed into E.coli JM109 host strain to obtain rHV3-Exendin-4 fusion protein expression engineered strain pTASHE / JM109.
Embodiment 3
[0025] Example 3 Expression of Hirudin III (HV3)-Exendin (Exendin-4) Fusion Protein in 7L Reactor
[0026] Inoculate a single colony of pTASHE / JM109 engineering bacteria in 200ml LB liquid medium (containing 100μg / ml ampicillin) at 37°C, 220rpm, and culture for 12h. Inoculate 5L fermentation medium (1% tryptone, 0.5% yeast powder, 4% sodium glutamate, 1% malt Powder, 0.671%KH 2 PO 4 , 0.757% Na 2 HPO 4 12H2O, 100 μg / ml ampicillin, pH 6.5), stirred culture at 37°C, the pH of the fermentation broth was controlled at 6.5-7.2 with phosphoric acid, and the dissolved oxygen was controlled at 40%-60%. When the fermentation broth OD 600nm When reaching 3.0 with about 30ml h -1 l -1 Feed medium I (10% malt powder, pH 6.5), the feed time is maintained for about 4 hours and the growth of the bacteria reaches the plateau stage. -1 l -1 Feed medium II (3.33% peptone, 1.67% yeast powder, 13.3% sodium glutamate, 10% malt powder, pH6.5) until the end of fermentation. The whole ferme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com