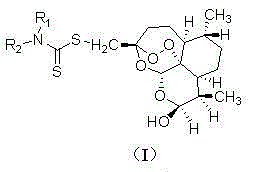
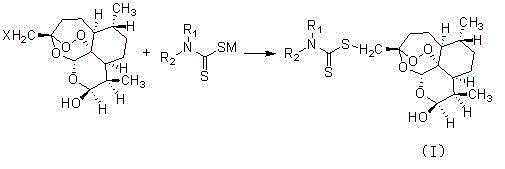
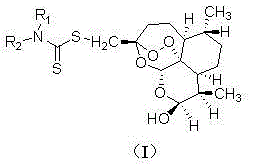
Dihydroartemisinin dithiocarbamate as well as preparation method and application of dihydroartemisinin dithiocarbamate
A technology of dihydroartemisinin carbamate and amino, which is applied in the field of dihydroartemisinin carbamate and its preparation and application, and can solve the problems of poor oral availability, short half-life, and water-soluble and poor fat solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (3R, 5aS, 6R, 8aS, 9R, 12S, 12aR)-octahydro-3-( N , N -diethylaminodithiocarboxy)methylene-6,9-dimethyl-3,12-oxo-12H-pyrano[4,3-j]-1,2-benzodithia Preparation of Ping-10(3H)alcohol (1)
[0019] The structure of compound (1) is shown below:
[0020]
[0021] Add 3.62 g (0.01 mol) of (3R, 5aS, 6R, 8aS, 9R, 12S, 12aR)-octahydro-3-bromomethylene-6,9-dimethyl-3,12 to the dry reactor -Oxo-12H-pyrano[4,3-j]-1,2-benzodithiapine-10(3H)alcohol and 25mL DMF, stirring, adding 2.05g (0.012mol) of N , N -Sodium diethylaminodithioformate, reacted for 12 hours, and evaporated the solvent under reduced pressure. Add 20mL ethyl acetate and 20mL water to the residue, stir, separate layers, extract the aqueous layer with ethyl acetate 15mLX2, combine the organic phases, dry, filter, concentrate, and purify by column chromatography to obtain the target compound (1), with a yield of 76% .
Embodiment 2
[0023] (3R, 5aS, 6R, 8aS, 9R, 12S, 12aR)-octahydro-3-( N -methylaminodithiocarboxy)methylene-6,9-dimethyl-3,12-oxo-12H-pyrano[4,3-j]-1,2-benzodithiapine Preparation of -10(3H)alcohol (2)
[0024] The structure of compound (2) is shown below:
[0025]
[0026] Add 3.18 g (0.01 mol) of (3R, 5aS, 6R, 8aS, 9R, 12S, 12aR)-octahydro-3-chloromethylene-6,9-dimethyl-3,12 to the dry reactor -Oxo-12H-pyrano[4,3-j]-1,2-benzodithiapine-10(3H)alcohol and 25mL isopropanol, stirred, added 1.55g (0.012mol) N -Sodium methylcarbamate and 0.82 g (0.0005mol) KI were reacted for 12 hours, and the solvent was distilled off under reduced pressure. Add 20mL ethyl acetate and 20mL water to the residue, stir, separate layers, extract the aqueous layer with ethyl acetate 15mLX2, combine the organic phases, dry, filter, concentrate, and purify by column chromatography to obtain the target compound (2), with a yield of 67% .
Embodiment 3
[0028] (3R, 5aS, 6R, 8aS, 9R, 12S, 12aR)-octahydro-3-[ N –(α-Methoxycarbonyl)methylaminodithiocarboxy]methylene-6,9-dimethyl-3,12-oxo-12H-pyrano[4,3-j]-1, Preparation of 2-benzodithiapine-10(3H)alcohol (3)
[0029] The structure of compound (3) is shown below:
[0030]
[0031] With 2.24g (0.012mol) N -Sodium (α-methoxycarbonyl)methylcarbamate instead N , N -Sodium diethylaminodithioformate, other operations are the same as in Example 1, to obtain compound (3), yield 79%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com